

KEY WORDS
Brain metastases, immunotherapy, NSCLC, poor predictive factors
INTRODUCTION
Non-small cell lung cancer (NSCLC) is a prevalent disease in the Netherlands, with over 10,000 new patients each year. In the past years, immunotherapy treatment with PDL1 inhibitors has become the standard of care for the majority of patients. Immunotherapy can result in both unusual responses and unusual toxicity. This has a major impact on patients as well as their treating physicians who must manage this new phenomenon. In this paper, we describe an unusual response to immunotherapy with poor predictive factors and also propose a better follow up for these patients.
CASE REPORT
In January 2017, 62-year-old healthy man was diagnosed with a symptomatic cerebral lesion of 5 cm in the left parietal lobe. His medical history only consisted of Graves’ disease with current hypothyroidism, stable on levothyroxine. He was a former smoker, who quit 28 years ago, after 15 pack years.
Further examination revealed a small tumour in the right upper lobe with a probable hilar lymph node metastasis and normal pulmonary function tests. Endobronchial ultrasound was negative. The patient was staged as T1cN0M1b NSCLC, oligometastatic. In February, he underwent a craniotomy. Pathological examination revealed a thyroid transcription factor 1-positive lung adenocarcinoma. Sequencing revealed wild type epidermal growth factor receptor (EGFR), KRAS, BRAF and ERB2. Anaplastic lymphoma kinase and ROS1 immunohistochemistry were negative. The completeness of the resection was doubtful; hence the patient was treated with additional stereotactic radiotherapy to his brain, 3 x 8 Gy.
After a quick recovery, he subsequently underwent a lobectomy of the right upper lobe. Pathological evaluation revealed a completely resected lung adenocarcinoma (3 cm), with a nodal metastasis on level 10R. Final staging was pT1cN1M1b. Thereafter, he underwent four cycles with adjuvant chemotherapy (carboplatin/pemetrexed), uneventfully.
In July 2017, he was doing well with a good quality of life. Thoracic computed tomography (CT) and magnetic resonance imaging (MRI) of his brain showed no tumour growth or new lesions. However, in April 2018, at a routine follow-up MRI of his brain, six new brain metastases were observed. He was subsequently treated with whole brain radiotherapy, 10 x 3 Gy. He was still in excellent health, with an Eastern Cooperative Oncology Group 0 performance status. He was treated for a short time with dexamethasone. A CT of the thorax and abdomen showed no signs of an extracranial recurrence.
Further treatment options were discussed. PDL1 testing on the lobectomy specimen revealed a PDL1 score of < 1% (Roche SP263 assay on a Ventana Benchmark Ultra). Apart from best supportive care, the treatment guidelines propose second-line chemotherapy or immunotherapy monotherapy. The PDL1 score is a mediocrely predictive marker for a patient’s response to immunotherapy.1 Our patient declined chemotherapy. Despite the low PDL1 score, we decided to start nivolumab immunotherapy in June 2018. After just two cycles of immunotherapy, he was admitted to the hospital with lethargy, nausea, and vomiting. Repeated MRI of his brain showed progressive disease, with increased leptomeningeal mass. A lumbar puncture was not performed.
Based on clinical and radiological deterioration, we defined the patient as having progressive disease. Nivolumab was stopped and dexamethasone was started at a dose of 2 mg b.i.d. After the start of Because of the progressive cerebral and leptomeningeal progression, the prognosis was deemed as very poor. For symptomatic relief, the dexamethasone was continued. The patient was discharged with best supportive care and no further appointments in the hospital were made. His general practitioner took over his care. dexamethasone, he quickly recovered and became asymptomatic. Further treatment options were considered. He again was not interested in treatment with docetaxel chemotherapy.
Because of the progressive cerebral and leptomeningeal progression, the prognosis was deemed as very poor. For symptomatic relief, the dexamethasone was continued. The patient was discharged with best supportive care and no further appointments in the hospital were made. His general practitioner took over his care. 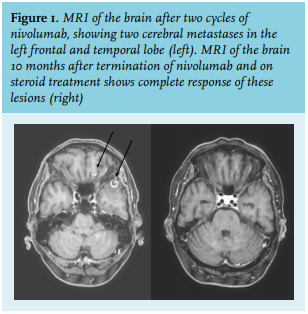
We concluded that the patient had experienced a delayed, near-complete response after just two doses of immunotherapy, despite a PDL1 score < 1% and continuous administration of 4 mg dexamethasone daily. The dexamethasone had caused Cushing’s syndrome, with severe weight gain, full moon face, and osteoporosis. Because of the estimated poor prognosis at progression (considered weeks), no osteoporosis prophylaxis and cotrimoxazol prophylaxis was started, neither was follow up of possible hyperglycaemia. He already used a proton pump inhibitor.
The patient was treated with additional laxatives, osteoporosis prophylaxis, and an orthopaedic lumbar support for pain relief, in addition to pain medication. The dexamethasone dosage was reduced and will be stopped after tapering. Further follow up with MRI is planned.
DISCUSSION
Nivolumab is one of the immunotherapy options in the second-line treatment of metastasised NSCLC, with a median overall survival of 12.2 months and 19% response rate.1 This effect is however, much stronger in patients with a high PDL1 expression compared to patients with low PDL1 expression. Patients with brain metastases could also potentially benefit from immunotherapy, as immunotherapy may cross the blood-brain barrier. In untreated brain metastases of NSCLC, pembrolizumab showed a 44% response rate in the brain in patients selected for PDL1 expression.2 Brain metastases may only decrease but may also show pseudoprogression after nivolumab and ipilimumab.3 Pseudoprogression is an increase in tumour size after start of immunotherapy, not caused by tumour growth but by a temporary inflammatory reaction of the tumour to immunotherapy. Pseudoprogression can be symptomatic, especially in the brain. In this case, we believe that the symptoms of our patient could be explained by pseudoprogression.
Of further note, it is known that the use of high-dose steroids has a poor impact on outcome.4 Patients receiving corticosteroids or its equivalent of > 10 mg daily for indications such as dyspnea or brain metastases had a decreased progression-free survival and overall survival. Interestingly, patients receiving corticosteroids for treatment of immune-related adverse events did not have a poorer treatment efficacy compared to patients not receiving corticosteroids.5
Altogether, we present a case of a patient with an exceptional and delayed cerebral response to two doses of nivolumab while on high-dose steroids. This case shows that immunotherapy can cause delayed and enduring responses even in patients with poor predictive parameters for treatment success, such as low PDL1 expression and long-term treatment with high dose corticosteroids. Of note, clinical and/or radiological pseudoprogression may also occur, causing a temporary deterioration, as in this patient.
This case also underscores the importance of follow up after the administration of immunotherapy, even in cases with a deemed poor prognosis and few received cycles of immunotherapy. This is equally important for the monitoring of side effects, which can evolve several months after the termination of immunotherapy.5 We believe that with a follow up, we may have prevented the development of steroid side effects, and that in hindsight, better prophylaxis and follow up for steroid side effect should have been started.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES