

KEY WORDS
Breast biopsy, breast ultrasound, clinical assessment, diagnosis, male breast cancer, mammography, triple assessment
INTRODUCTION
Male breast cancer is a rare malignancy comprising less than 1% of all male cancers and is not completely understood because most of the available data is obtained from single institution studies with no statistical significance.1 Traditionally, male breast cancer was thought to be similar to post-menopausal female breast cancer, but emerging evidence suggests that male breast cancer may be different.2
From the clinical and biological points of view, male and female breast cancers differ primarily in the frequency of their histological types and in the expression of hormone receptors (oestrogen receptor (ER) and progesterone receptor (PR)) and the human epidermal growth factor receptor 2 (HER2).3
DISCUSSION
The diagnosis of male breast cancer can made in most cases by triple assessment: clinical assessment, radiologic assessment (mammography and ultrasound examination), and tissue biopsy (fine-needle aspiration cytology or core biopsy), exactly as in female breast cancer.
Clinical assessment
The most common symptom of breast cancer is a painless retroareolar mass which is present alone or with other symptoms in 75% of all cases. The mass is found more frequently on the left more than on the right,4 and pain is present with the mass in 5% of all patients.5
Because of the smaller size of breast tissue in males, nipple involvement is seen early in the course of the malignant process, with ulceration in 6% of the patients, discharge in 6%, and retraction in 9%. When there is only bloody nipple discharge without a palpable mass, the diagnosis can be made early and is usually ductal carcinoma in situ of low or intermediate grade.6 Paget’s disease is rare, presenting in only 1% of patients, with a mean age of onset of 60 years, similar to that of other males with breast cancer.7
Axillary lymph node involvement at presentation is more common in men than in women. In rare cases, male breast cancer presented as an occult primary with lymph nodes metastases.8 In summary, clinical presentation extends a spectrum of the most common painless breast mass, or mass with variable symptoms, to the least common metastatic disease.
Clinical evaluation should look specifically for male breast cancers risk factors which are divided into high risk groups such as advanced age, antiandrogens therapy, radiotherapy and hormonal imbalance as in liver failure, and strong family history due to BRCA 2 mutations.9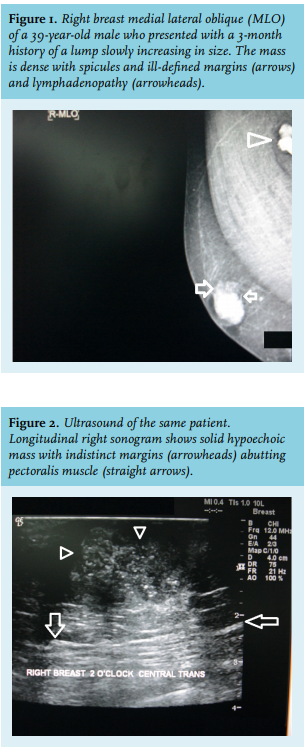
The tumour-node-metastasis staging system may not be suitable in male breast cancer because of scarce breast tissue and early chest wall and lymphatic spread.12 However, it is common practice to stage male breast cancer by computed tomography scan of the chest, abdomen, and pelvis.
Radiologic assessment
Mammography can identify malignant breast tumours with a sensitivity of 92-100% and a specificity of 90%. Axillary ultrasound could be helpful in staging since a significant portion of patients have axillary metastases at diagnosis.13
A male breast cancer mass may have spicules and irregular margins (figure 1) and can be readily recognised from the surrounding tissue and eccentric position. Microcalcifications are not as common in male breast cancer as in their female counterparts.14 Skin retraction or ulceration and lymph node involvement are suggestive of male breast cancer.
Male breast ultrasonography shows malignant lesions as solid lesions (figure 2) or complex cystic lesions, both of which mandate biopsy.15 Ultrasound can be 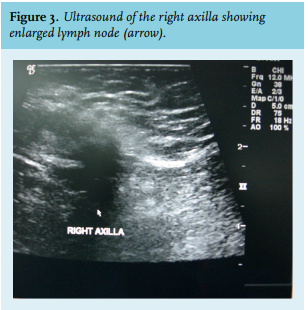
Tissue biopsy
Core biopsy is preferred over fine-needle biopsy because it enables a definitive diagnosis of invasive breast cancer. The presence of malignant cells on a cytology specimen may be the result of ductal carcinoma in situ rather than invasive disease, and treatment of the two diseases is completely different. In addition, core biopsy yields a tissue sample similar to that of open biopsy without the need for a formal surgical procedure.
Histopathology
The most common histological type of male breast cancer is invasive ductal carcinoma, which represents more than 90% of all cases.16 The other histopathological types of male breast cancer from the most to the least common include ductal carcinoma in situ, adenocarcinoma not otherwise specified (NOS), invasive papillary carcinoma, carcinoma NOS, medullary carcinoma mucinous carcinoma, inflammatory carcinoma, phyllodes tumour, leiomyosarcoma, Paget’s disease, and lobular carcinoma.
The rudimentary male breast tissue lacks the terminal lobules and does not differentiate into lobules unless under the influence of high concentrations of endogenous or exogenous oestrogen. The lobular histotype accounts for only 1.5% of invasive cancers. Lobular carcinoma has been reported in men with Klinefelter’s syndrome2 and surprisingly, in genotypically normal males with no previous history of oestrogen exposure.17
Ductal carcinoma in situ of the male breast cancer represents 10% of all cases. Most of these (74%) are papillary carcinomas, usually of low or intermediate grade.6 All the histological subtypes noted in females are also seen in males; the most prominent patterns being the papillary and cribriform patterns. Much rarer tumour types include invasive papillomas and medullary lesions.
The invasive male breast cancers are histologically classified according to the World Health Organisation 2012 criteria. The frequency of histological subtypes differs between males and females, with invasive carcinomas of no special type being by far, the most common subtype (> 90%), followed by invasive papillary carcinoma and invasive micropapillary carcinomas.2
Oestrogen receptor positivity has been reported in more than 90% of male breast cancers, with 92–96% being progesterone-receptor positive.18
Surprisingly, male breast cancers are more hormone receptor-positive than female breast cancers (ER > 90& vs 76% and PR > 75% vs 60%, respectively).19 The proportion of the male breast cancer expressing the hormone receptors increases with age as in post-menopausal women.3
HER2 amplification is seen in less than half as frequently in male breast cancer when compared to female breast cancer (5% vs 15%). Subsequently, the most common phenotype seen in male breast cancer is the luminal (ER+ and/or PR+, HER2-) subtype.2
BRCA2 mutations are found in 4-40% of male breast cancers according to different studies. The most common pathological type in those patients is invasive ductal carcinoma. BRCA2-related male breast cancers showed a significant association with HER2 over-expression and negative PR. This might be an additive factor to more aggressive behaviour if the tumour was also high grade.19
CONCLUSION
The diagnosis of male breast cancer should follow simple orderly steps similar to female breast cancer diagnosis. Careful attention and good evaluation for breast complaints, especially in high-risk patients, is essential to avoid misdiagnosis. Increased public awareness about the presence of such a cancer in men is warranted to promote seeking care for breast changes in males. The most common clinical picture is a hard retroareolar mass in the left breast in a man in his sixth decade, which is suspicious upon mammography and ultrasonography; its most common pathology is invasive ductal carcinoma in stage II or III.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES