

KEYWORDS
Adult-onset Still’s disease, platelets, platelet distribution width, sepsis
INTRODUCTION
Adult-onset Still’s disease (AOSD) is a rare, chronic, and systemic inflammatory disorder.1 It was first described in 1971, but the etiopathogenesis of the disease is still unclear.2 The incidence of AOSD can reach to 0.16-0.4 per 1,000,000 persons.3 Fever is a main sign of patients with AOSD.4,5 Fever is also common in sepsis.6 Diagnosis of patients with AOSD or sepsis is exhausting, time-consuming, and costly.7 Differentiating these two clinical diseases with a simple and easily available marker is invaluable; however, there is no objective marker to distinguish AODS from sepsis.
Several reports have suggested that platelets (PLTs) contribute to antimicrobial defence during various acute and chronic infections.8,9 It might be beneficial when PLTs, plateletcrit (PCT), mean platelet volume (MPV), and platelet distribution width (PDW) are combined with other acute phase reactants to demarcate inflammatory diseases.10,11 The objective of this study is to evaluate whether PLTs, PCT, MPV, PDW, and the PLT-to-PDW ratio (PPR) are reliable for distinguishing diagnosis of AOSD from sepsis.
METHODS
Patients
This retrospective cohort study was carried out in a tertiary hospital. Patients newly diagnosed with AOSD between 2010 and 2018 in The First Affiliated Hospital of Nanjing Medical University were recruited. The diagnosis of AOSD was confirmed by the Yamaguchi criteria.12 For the present analysis, only adult patients ≥ 18 years were included, but patients who had cancer, used anticoagulants prior to admission, and had no PDW values were excluded; 82 patients were included as AOSD. Simultaneously, 48 age-and gender-matched patients with a systemic inflammatory response syndrome accompanied by proven microbial infection were included as sepsis. Patients who met two or more of the following conditions were defined as sepsis: (1) temperature > 38°C or < 36°C; (2) heart rate > 90 beats per minute; (3) respiratory rate > 24 breaths per minute or partial pressure of carbon dioxide < 32 mm Hg; (4) white blood cell count > 12×109/l, < 4×109/l, or > 10% immature forms.13,14 Seventy-six age- and gender-matched healthy controls were also included.
Ethical statement
The study protocol was performed to conform with the Declaration of Helsinki and was approved by the local ethics committee of the hospital. This article does not contain any studies with animals.
Data collection
Patients’ age, gender, clinical features (fever, arthritis, myalgia, rash, sore throat, lymphadenopathy, pericarditis, hepatomegaly, splenomegaly, pleuritis, pneumonitis), and clinical data (organism, C-reactive protein (CRP), ferritin, whole blood counts (WBC), lymphocyte count, neutrophil count, PLTs, PCT, MPV, and PDW, which were measured from peripheral venous blood samples at admission) were collected.
WBC; neutrophil, lymphocyte, and platelet counts; and platelet parameters (PCT, MPV, and PDW) were analysed using Sysmex XE 2100 analysers (Sysmex, Hyogo, Japan). CRP level was performed by a BN II nephelometer (Dade Behring, Marburg, Germany). Ferritin analysis was performed on a Unicel DXI 800 (Beckman Coulter, Brea, CA, America). All blood samples were taken on the day of admission and measured within two hours. PPR was calculated by dividing the platelet count by the PDW.
Statistical analysis
Statistical analysis was performed using SPSS 21 software (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as mean and standard deviation or median and range. The Kolmogorov-Smirnoff test and Levene’s test were used to assess the normality of the distribution and the homogeneity of variance. Variables were compared by the independent sample t-test or one-way ANOVA test and the nonparametric Kruskal-Wallis test, depending on distribution form. The Fisher Exact test was used for categorical variables. Spearman correlation was used when variables were normally distributed and the data of descriptive analysis were expressed as mean ± standard deviation (SD). Pearson correlation was used when variables were non-normally distributed and the data were expressed as the median, interquartile range. Non-collinear and significant variables in the univariate analyses were included in multivariate logistic regression analyses. The optimal differential diagnosis values of the laboratory variables were identified by receiver operating characteristic curves (ROC) and quantified by calculating the area under the ROC curve (AUC). A p-value < 0.05 was considered statistically significant. All p-values were from two-sided tests.
RESULTS
Demographic and clinical features of study patients
Clinical characteristics of all patients are described in table 1. Median age of AOSD patients (male/female: 29/53) was 36 (18-74) years old, that of sepsis patients (male/female: 25/23) was 40 (18-78) years old, and that of healthy controls (male/female: 31/45) was 39 (18-75) years old. There were no significant differences in the median age and gender among the three groups. The most common clinical manifestation of AOSD patients was fever (100%), followed by typical rash (50%), sore throat (45.1%), arthralgia/arthritis (42.7%), and myalgia (35.3%). The main clinical symptoms in sepsis included fever (100%), arthralgia (10.4%), s kin rash (6.3%), and sore throat (8.3%). Bacteraemia was verified in 21 (43.6%) sepsis patients. Nineteen (39.6%) sepsis patients had pneumonia, 11 (22.9%) had a urinary tract infection, and 6 (12.5%) had hepatobiliary infection.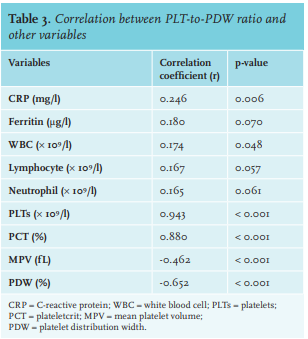
Table 2 shows the clinical data of the patients between the two groups. The results suggest that PPR and ferritin were significantly higher in the AOSD group compared with sepsis patients (22.18 ± 11.12 vs. 13.80 ± 8.97, p < 0.001; 3972.90 ± 5134.04 μg/l vs. 518.92 ± 382.50 μg/l, p = 0.001, respectively). In addition, the median PPR in both AOSD and sepsis groups was significantly higher than that in healthy controls (figure 1). The CRP of AOSD and sepsis groups was 106.94 ± 69.22 mg/l and 72.13 ± 56.69 mg/l, respectively, and significantly higher in the AOSD group (p = 0.005). A significantly higher PCT level was observed in the AOSD group than in the sepsis group (0.25 ± 0.10 vs. 0.19 ± 0.09, respectively, p = 0.002). Both lymphocyte count and PLTs were higher in the AOSD group than in sepsis group (p =0.096, p < 0.001, respectively); MPV and PDW were lower in the AOSD group than in sepsis group (all p < 0.001).
PPR was positively correlated with CRP (r = 0.25), WBC (r = 0.17), PLTs (r = 0.94), lymphocytes (r = 0.17), neutrophils (r = 0.17), ferritin (r = 0.18), and PCT (r = 0.88), whereas it was negatively correlated with MPV (r = -0.46) and PDW (r = -0.65). PPR showed no significant correlation with lymphocytes (p = 0.057), neutrophils (p = 0.061), or ferritin (p = 0.070) (table 3).
In the multivariate analysis (which included ferritin, lymphocytes, MPV, PDW, and PPR) only PPR and ferritin remained independent factors to differentiate AOSD from sepsis (OR: 5.86, 95% CI 1.59 - 21.60, p = 0.008; OR: 54.06, 95% CI 9.57 - 305.44, p < 0.001; respectively) (table 4).
PPR could be used to distinguish AOSD from sepsis
We further evaluated the identification value of PPR and ferritin in AOSD and sepsis by constructing ROC curves (figure 1). The ROC analysis showed that the AUC of PPR and ferritin were 0.733 (95%CI 0.646-0.820, p < 0.001) and 0.887 (95%CI 0.825-0.950, p < 0.001), respectively. The best cutoff value of PPR was 16.8, with a sensitivity of 61.0% and specificity of 68.0%. With a cutoff value of 1120 μg/l, the sensitivity and specificity of ferritin were 74.7% and 88.9%, respectively. Moreover, the combination of PPR and ferritin yielded a higher AUC value at 0.931 (95%CI 0.884-0.984, sensitivity: 84.0%, specificity: 92.6%). The results indicate that PPR has individual identification value and the combined identification value of PPR and ferritin is much higher than that of any single factor.
DISCUSSION
In this study, we found that PPR in both groups (AOSD and sepsis) was significantly higher than that in healthy controls and it was an independent factor for differential diagnosis of AOSD and sepsis. The value of the combination of PPR and ferritin to differentiate between AOSD and sepsis was much higher than for each of them alone. Therefore, PPR and the combination of PPR and ferritin would be favourable in clinical practice for differential diagnosis of AOSD from sepsis.
Several biomarkers, such as interleukin-6, procalcitonin, and hyperferritinaemia had been investigated between AOSD and sepsis.15-18 However, a definite biomarker had not yet been established clinically as they might emerge during inflammatory diseases.19 Clinical manifestation could also differentiate AOSD from sepsis.20 We compared clinical characteristics between two groups and the results showed that arthritis, skin rash, sore throat, and myalgia could distinguish them, but symptoms are nonspecific, easily ignored, and may appear at different phases of the disease. In addition, recall bias may be possible.
Therefore, a useful marker is extremely needed to discriminate AOSD from sepsis. Here, the novel marker PPR is becoming useful. In the current study, we first confirmed that PPR, ferritin, CRP, PCT, lymphocyte count, and PLTs increased in AOSD patients, and that MPV and PDW decreased in the AOSD group. Then we ascertained in the current model that PPR and ferritin were the only independent factors able to distinguish AOSD from sepsis, which indicates that ferritin and PPR are more stable than other variables, including PDW. According to the results of ROC curves, we further proved that PPR and ferritin were able to distinguish between AOSD and sepsis.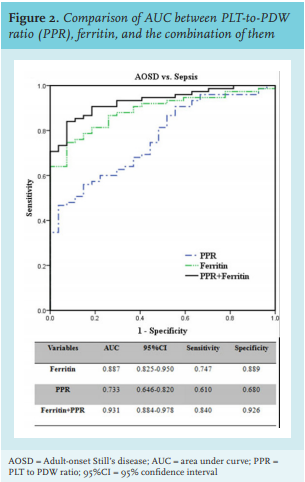
There are reports of combining biomarkers to improve the differential diagnosis accuracy of AOSD.23,24 In our study, we also investigated whether the combination of PPR and ferritin could distinguish between AOSD and sepsis. The result showed that the AUC of the combination was 0.931 (0.884-0.984), and that the combined sensitivity and specificity of PPR and ferritin was better than that of PPR or ferritin alone. The values of sensitivity and specificity of the combined differential diagnosis of our combination (PPR and ferritin) are much higher (sensitivity: 84.0% vs. 43.2%, specificity: 92.6% vs. 88.9%) when compared with the combination of ferritin and glycosylated ferritin.24 In addition, as part of the complete blood count test, PLTs and PDW can be measured by blood cell analysers when patients with a fever are admitted to hospital, enabling calculation of PPR. Therefore, PPR could be a helpful marker for physicians to discriminate AOSD from sepsis as objective laboratory biomarkers are lacking.
There are some limitations in our study. First, we retrospectively reviewed the clinical data and some patient data may be not available. Second, as a single centre study, there may have been selection bias. Third, due to methodological and financial reasons, we could not include potential biological markers that can differentiate AOSD from sepsis, such as interleukin-18 and serum amyloid A.25,26 Therefore, more prospective, multi-centre, multi-population, multi-index research is needed.
In summary, both PPR and ferritin were able to independently identify AOSD from sepsis, and the combined diagnosis value of PPR and ferritin is much higher. In addition, PPR and ferritin are easy-to-obtain markers. Therefore, PPR might be useful for differentiating AOSD from sepsis as a supplementary variable to ferritin.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES