AbstractFull textPDF
Full text
KEYWORDS
Cellulitis, clinical management, pathophysiology, review
INTRODUCTION
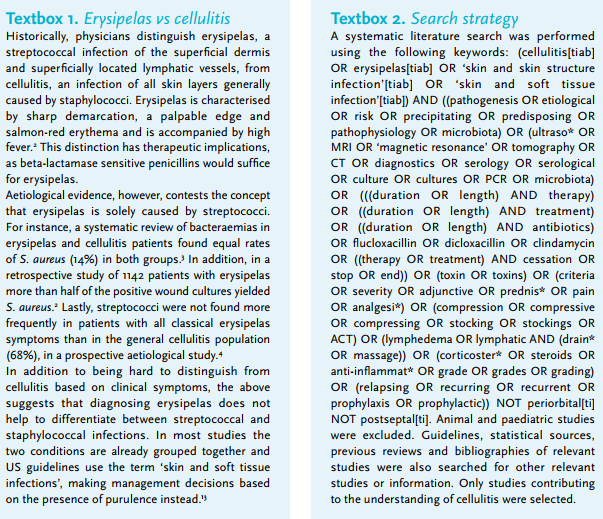
Cellulitis (Latin: cellula (diminutive of cella: cell) + itis (suffix denoting inflammation)) and its subtype erysipelas (Greek: erythrós (red) + pella (skin)), are among the most frequent infections requiring hospitalisation.1 The historical distinction between cellulitis and erysipelas, based on different bacterial aetiologies and thus treatment options, is becoming obsolete as increasing evidence suggests a large overlap between these two entities (textbox 1). In the Netherlands, the annual incidence is estimated to be 22 per 1000 inhabitants. Approximately 7% of all patients with cellulitis are hospitalised.5,6 The mortality rate of hospitalised patients has been reported to be around 2.5%.7 Recent epidemiology data on cellulitis in the Netherlands are lacking, but given the rise in the incidence of important risk factors (namely diabetes, obesity and old age), an increase in the incidence of cellulitis is expected.8-10 Dutch guidelines on the clinical management of cellulitis of the lower extremities have been available since 2013 (figure 1).11 Since their publication, numerous studies have provided novel insights and new antibiotics registered for skin and soft tissue infections have entered the market. This review discusses the current state of evidence regarding pathogenesis, diagnostics, and treatment of cellulitis. The literature search strategy used is documented in textbox 2.
Cellulitis: a diagnostic challenge
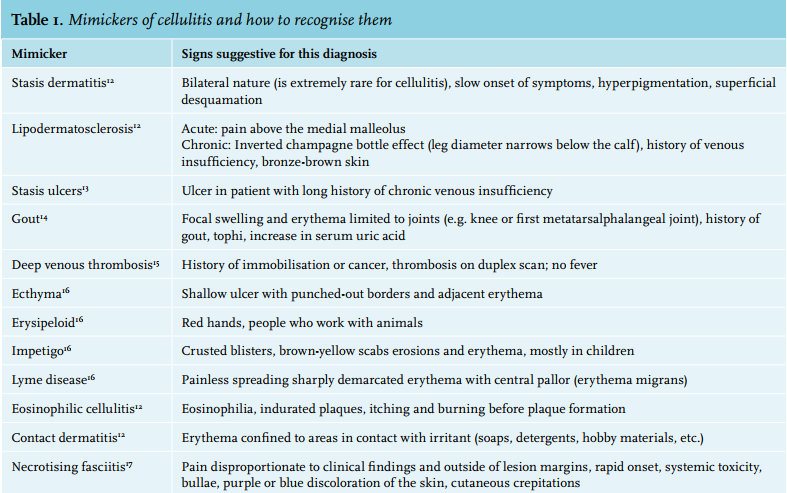
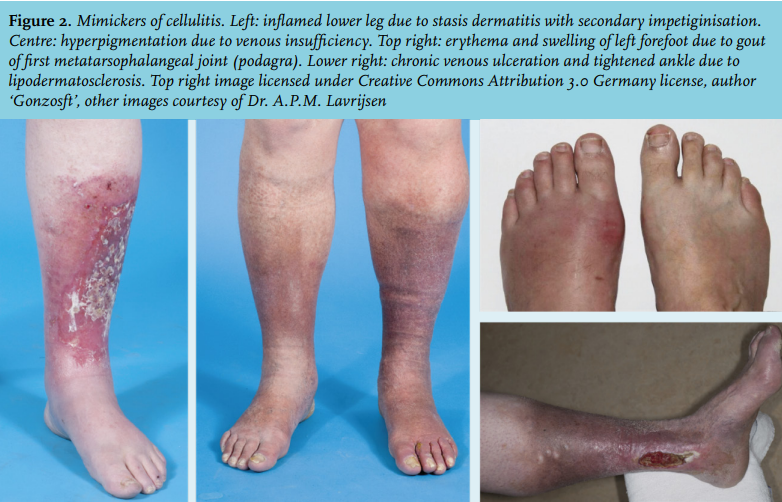
All that is red is not cellulitis. The classical symptoms of erythema, oedema, warmth and tenderness, are non-specific and vary in severity. The clinical presentation of cellulitis is mimicked by a whole range of diseases (table 1 and figure 2). One recent study revealed that 31% of patients hospitalised with cellulitis were misdiagnosed, the most frequent mimickers being stasis dermatitis, stasis ulcers, gout, congestive heart failure, non-specific oedema and deep venous thrombosis (DVT).18 Another study in the primary care setting found a similar rate of misdiagnoses.19 Furthermore, when clinicians specifically consulted dermatologists because of uncertainty about a diagnosis of cellulitis, 74% of the patients turned out not to have cellulitis.20 Misdiagnosis results in unnecessary admissions and extra costs for perceived refractory cellulitis.18 Leucocytosis and elevated C-reactive protein (CRP) levels are present in 34-50% and 77-97% of patients, respectively.21,22
Stasis dermatitis can mimic all symptoms, including mild leucocytosis and/or CRP elevation. Its origin lies in chronic venous insufficiency, which causes proliferation and increased permeability of dermal capillaries. Leucocytes migrate, cause inflammation, stimulate collagen production, and thus induce dermal fibrosis.23 Erythrocyte extravasation causes brown skin pigmentation.24 Untreated, stasis dermatitis can progress to lipodermatosclerosis, which is characterised by a fibrotic tightening and sometimes ulceration of the skin above the ankles. Compression therapy can correct haemodynamic effects and cytokine levels.13
Imaging is sometimes indicated. As DVT does not occur more often in patients with cellulitis than those without, routine DVT screening is not recommended.25 When ultrasound was only utilised among uncertain ‘DVT vs cellulitis’ diagnoses, 17% turned out to have DVT.26 Ultrasound may detect occult abscesses, or disprove ‘abscesses’ mistakenly diagnosed during physical examination.27 Computed tomography is not warranted due to nonspecific findings.28 Magnetic resonance imaging and the Laboratory Risk Indicator for Necrotising Fasciitis score might help distinguish between necrotising fasciitis and cellulitis, but as yet neither have proven superior to clinical suspicion and subsequent surgical exploration.29 Uncomplicated superficial abscesses with erythema can be difficult to distinguish from primary cellulitis with secondary abscesses.30 Uncomplicated abscesses are treated with incision and drainage,31 but two recent trials show cure rate increases from 69-74% to 81-83% with adjunctive antibiotics.32,33
Risk factors
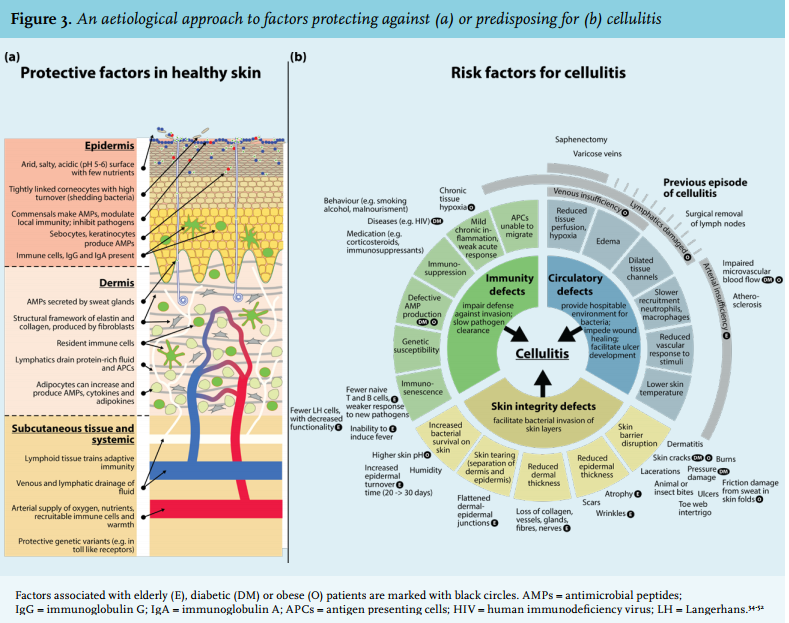
Multiple physical barriers and active protective mechanisms prevent the invasion of skin commensals and thus the occurrence of infection (figure 3a). An intact vasculature will help maintain the integrity and function of all these barriers and mechanisms. Deficiencies in skin integrity, immunity or vasculature can be considered risk factors for the development of cellulitis (figure 3b). Old age, diabetes and obesity cause defects in all three of these areas, and thus confer a relatively high risk. This combination of risk factors is often seen in patients with cellulitis who are hospitalised. The biggest risk factor, however, is a positive history for cellulitis.34
Old age comes with skin atrophy, poor circulation, immunosenescence, and comorbidities such as diabetes or congestive heart failure. Malnourishment causes impaired wound healing, decreased skin elasticity and integrity, and relative immunosuppression.35 Incidence, complication (e.g. bacteraemia, osteomyelitis, endocarditis) and hospitalisation rates are all higher in diabetic patients.53 Most cases of cellulitis in diabetic patients will be attributable to diabetic foot associated skin defects, but more than a quarter of the cases of culture-positive diabetic cellulitis occur on non-foot locations.54 In morbid obesity, the skin is more susceptible to damage and takes longer to repair.36
Some seasonal variability has been observed. Streptococcal skin infections occur more frequently in the winter in cold countries,55,56 while warmer regions see a higher erysipelas incidence during the summer.37
Skin microbiome alterations have been observed in diseases such as atopic dermatitis, where more staphylococci and fewer streptococci are present, but also in acne.57 S. aureus is shown to be overrepresented in the peri-abscess skin microbiome.58 Pioneering studies have revealed that commensals can influence the composition of the local microbiome and alter local immunity,59 but future studies will have to reveal relationships between the microbiome and cellulitis.
To admit, or not to admit
The 7% of patients who are hospitalised cause 83% of the total healthcare expenditure associated with cellulitis.6 Unfortunately, as yet there are no validated, prospectively evaluated admission guidelines. One system distinguishes classes with supposedly increasing mortality and therapy failure rates based on systemic symptoms, comorbidity and the Standardised Early Warning Scores.60,61 Two cohort studies compared this system with current clinical practice: one retrospectively, one prospectively.62,63
Overtreatment of infections that the system classified as mild (class I and II) was very common, while most of the severest infections (class IV) were undertreated. In one of the two studies, only 5 of 6 (83%) class IV patients had achieved complete resolution of symptoms at the end of therapy, compared with 100%, 98% and 96% in classes I-III.62,63 One explanation for this is that factors not incorporated in this system currently have a substantial effect on admission and treatment practices.
Pragmatically, one could consider admission for patients with (1) poor disease perception, (2) intake problems, (3) an altered mental status, or (4) disease progression despite adequately dosed oral antibiotics. Severity or dysregulation of comorbidity (e.g. diabetes, immunodeficiency, obesity, or cardiac, renal or venous insufficiencies) and severity of infection (e.g. systemic symptoms, organ failure) should also be taken into account.11,64
Factors predicting oral therapy failure may also be indications for admission for intravenous antibiotics. Retrospectively identified factors associated with failure of oral antibiotic therapy include fever, chronic leg ulcers, chronic oedema and lymphoedema, prior cellulitis in the same area, and wound infections.65,66 Additionally, after treatment in an observation unit for 24 hours, patients with cellulitis of the hand, an elevated lactate, fever, history thereof, or multiple comorbidities were more likely to be admitted.67,68 However, this mainly reflects clinical practice rather than need for admission.
Alternatively, outpatient parenteral antibiotic therapy, where intravenous antibiotics are given at home or on outpatient basis, can avoid or shorten hospitalisation for selected patients and is usually preferred by patients.69
Antibiotic treatment
One might wonder if a proportion of cases of cellulitis are self-limiting and do not require antimicrobial agents. It is noteworthy that in clinical trials performed in the pre-antibiotic era, in which the effects of horse serum and ultraviolet light were evaluated, cure rates of 70% were observed.70 On the other hand, it has also been demonstrated that inadequate empirical antibiotics are associated with prolonged treatment durations and length of hospital stay.71 Current treatment recommendations are summarised in figure 1.
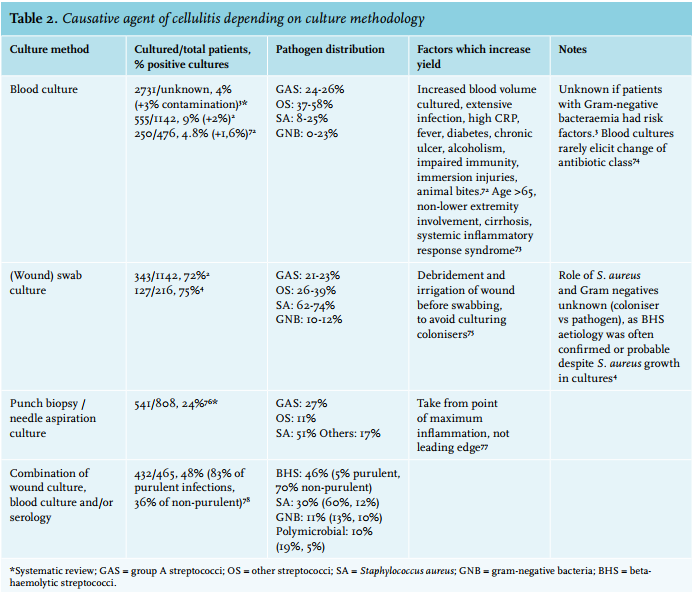
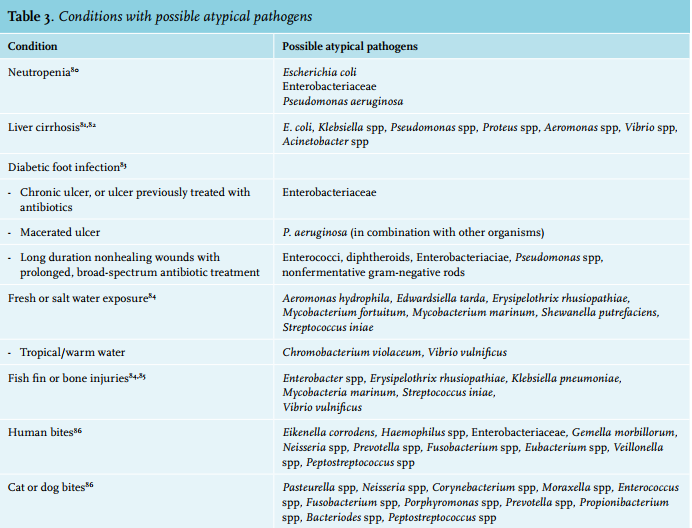
Streptococci and S. aureus are the most common pathogens identified in patients with cellulitis (table 2), and accumulating evidence from prospective convalescent serology studies suggests that > 70% are caused by streptococci.4,79 Atypical pathogens can be observed in patients with selected conditions (table 3). In contrast to diabetic foot infections, diabetic non-foot infections are generally not caused by atypical pathogens.87 In the Netherlands, the preferred small spectrum agent covering both methicillin-susceptible S. aureus and beta-haemolytic streptococci is flucloxacillin. Confirmed streptococcal infections can be treated with benzylpenicillin or feneticillin. Co-amoxiclav and clindamycin are alternative options. Clindamycin is recommended in case of beta-lactam allergies, and inhibits streptococcal and staphylococcal toxin production. Clindamycin is also thought to have better tissue penetration than beta-lactams. However, clindamycin is highly concentrated intracellularly, and studies measuring tissue concentrations used homogenised tissues and thus also measured intracellular clindamycin.88 This overestimates relevant clindamycin levels in the extracellular fluid, while the primarily extracellular beta-lactam concentration is diluted by the released intracellular volume and thus underestimated.88 Of note, some S. aureus strains have inducible resistance for clindamycin, showing growth inhibition in vitro but resistance in vivo.89,90 In the Netherlands, around 10% of S. aureus from selected general practice patients and hospital patients show (inducible) resistance to clindamycin, compared with less than 3% for flucloxacillin.90 This makes clindamycin less preferable as an empirical choice.
Evidence does not favour one agent over others, although there is a major lack of evidence in this area.91 One study found pristinamycin to be slightly more efficacious than penicillin in a non-blinded trial, but did not account for penicillin not covering S. aureus. 3,92 Beta-lactams were as effective as non-beta-lactams in a cohort study.93 A recent meta-analysis comparing penicillins or cephalosporins with macrolides or lincosamides (such as clindamycin) found similar efficacy between the two groups.94
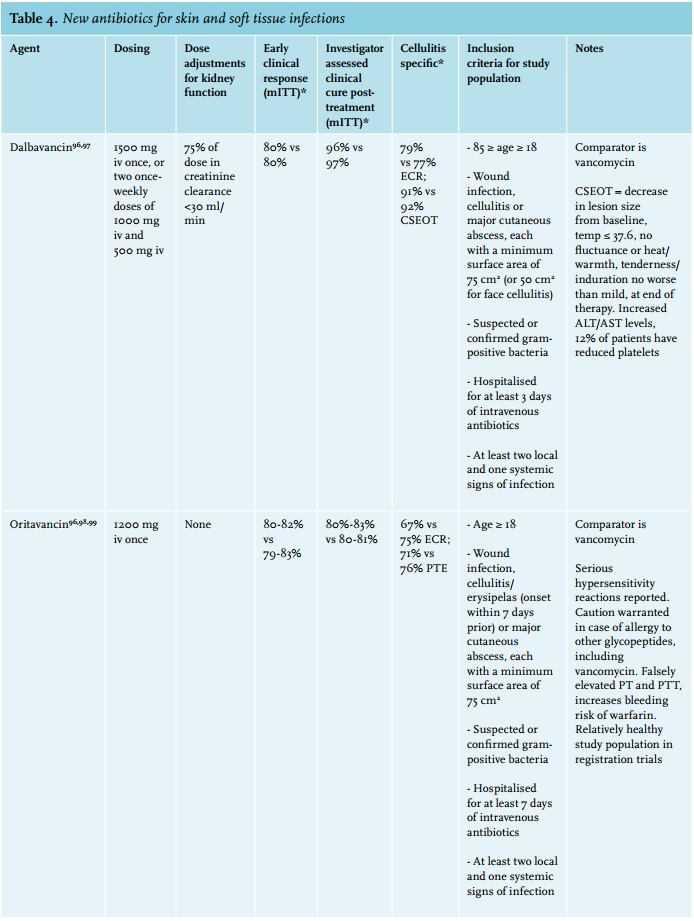
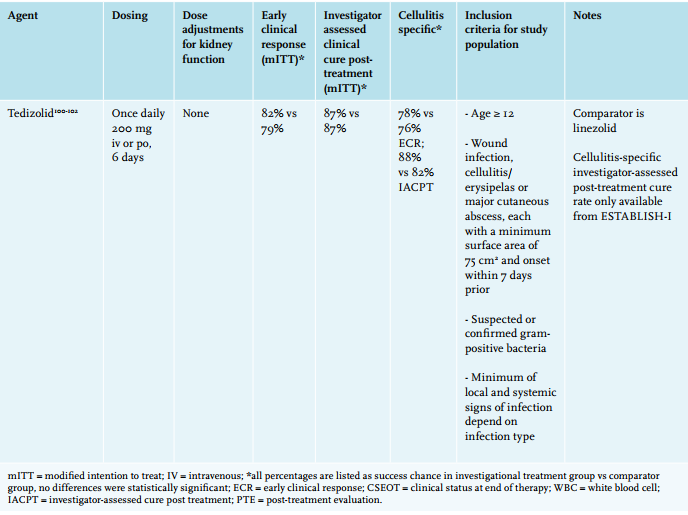
If one needs to cover multi-resistant Staphylococcus aureus (MRSA), vancomycin remains the first choice of treatment, with linezolid as an alternative.95 Additionally, three novel antibiotics have recently been approved by the European Medicines Agency for treatment of skin infections: oritavancin and dalbavancin, two (lipo)glycopeptides, and tedizolid, an oxazolidinone, all showing potent activity against MRSA similar to vancomycin and linezolid (table 4).96 Oritavancin and dalbavancin both have terminal half-lives of over two weeks and thus only require a single intravenous dose to reach cure rates non-inferior to a 2-week course of vancomycin.98,103 Whether this actually reduces the number of admissions or total treatment costs remains to be evaluated.
Optimising antibiotic use
For oral flucloxacillin, proper timing of intake (before or long after meals) optimises the bioavailability to ~55%.104 Beta-lactams reach lower serum concentrations in obese patients due to altered distribution volumes and clearance, so these patients might benefit from higher oral dosing, or more frequent intravenous dosing.105 This is underscored by the fact that obese patients tend to have lower cure rates.106,107
The optimal duration of antibiotic treatment of cellulitis is unknown. One study suggested that patients with cellulitis who are treated on an outpatient basis only require 5 days of therapy when signs of improvement are seen.108 However, this study used unconventional numbers for its power calculation, had a dropout rate of 30% before randomisation due to non-improvement, included relatively young and healthy subjects and made use of levofloxacin as study drug.11 Community-acquired pneumonia, pyelonephritis and intraabdominal infections require shorter antibiotic treatments than we previously thought necessary.109-111 Whether cellulitis treatment can also be shortened is under investigation.112
Some patients have an increased risk of a complicated infection. Obesity predisposes to local complications such as bullae, abscess formation, haemorrhagic lesions and necrosis.113,114 Smoking and delays in antibiotic treatment are also linked to abscess formation.113 Patients with congestive heart failure, neutropenia, hypoalbuminaemia, an altered mental status or discharge from the lesion have an increased risk of experiencing adverse outcomes, in terms of death, local complications (e.g. requiring surgical drainage) or systemic complications (e.g. multi-organ failure).7
Non-antibiotic management
Additional non-antibiotic management options can potentially improve outcomes. Compression therapy has long been, and still is, cause for debate. Advocates claim there is an accelerated reduction of oedema and pain, and shorter time to cure. Currently, no evidence supports this claim. Patients, however, often report side effects such as pain, dry skin, itching, constriction and slipping.115,116 It is unknown if the altered haemodynamics affect the time to microbiological cure. The adequacy of applied bandages varies in clinical practice, and inadequately applied bandages can cause pressure ulceration, thus unnecessary harm.117,118 An alternative to reduce oedema in the acute phase is passive leg elevation. To prevent persisting lymphoedema from causing recurrences, compression therapy is indicated when lymphoedema persists for several weeks after antibiotic treatment.11 Compression stockings should follow initial bandaging, provided the patient’s arterial disease status allows it.118
The use of anti-inflammatory drugs in addition to antibiotic therapy might be beneficial. In a proof-ofconcept study, adjunctive non-steroidal anti-inflammatory drugs (NSAIDs) led to faster regression and resolution of symptoms.119 Similarly, patients receiving adjunctive oral prednisone (2 days 30 mg, 2 days 15 mg, 2 days 10 mg, 2 days 5 mg) had earlier resolution of symptoms and intravenous to oral antibiotic switches.120,121 Whether these drugs also affect microbial eradication is as yet unknown.
Recurrent cellulitis
Almost 30% of admissions for cellulitis are for recurrent cellulitis.122 Two, three and five year recurrence rates are 17%, 29-47% and 47%, respectively.38,123-125 Five-year recurrence rate is 57% in patients with a history of recurrence.125 For HIV-infected patients, one- and three-year recurrence rates are 29% and 47%.126 Independent of persisting risk factors that might explain recurrences, the first episode’s inflammation has also likely damaged local lymphatic channels. Drainage is then insufficient, antigen presenting cells cannot migrate, and accumulating protein-rich fluid accommodates invading bacteria.127
Lymphoedema is the most important risk factor for recurring cellulitis, and 25-60% of recurrent cellulitis patients suffer from chronic oedema.122,124 Obese patients have more recurrences and CRP and leucocyte counts are higher in these recurrences.39 For HIV-infected patients specifically, non-hepatitis liver disease, intravenous catheters or intravenous drug use increase the recurrence risk.126 All persisting risk factors are also likely to increase the chance of recurrences, and should be treated vigorously when possible. Lymphoedema warrants treatment with compression therapy. Tinea pedis should be treated with topical azoles in order to decrease the chance of recurrence. Frequent and meticulous interdigital web space cleansing prevents skin damage, bacterial overgrowth and bacterial invasion.127
S. pyogenes is able to survive and replicate within macrophages.128 Theoretically this might elicit recurrences. However, recurrence rates are similar between patients receiving antibiotics with or without intracellular activity.129 When infections recur despite adequately treating risk factors, prophylactic antibiotics prevent recurrences.130 In the PATCH I trial, which randomised 274 patients with two or more episodes to either twice daily low-dose oral penicillin or placebo, recurrence rates were significantly lower in the penicillin group (22% vs 37%) after one year, although this effect wore off after cessation of treatment.131 For recurring S. aureus infections, on-demand therapy can be considered. S. aureus eradication or hygiene measures do not prevent recurrent S. aureus skin infections.132,133
Future perspectives
An overview of knowledge gaps which, if addressed, could advance our understanding of the pathophysiology of cellulitis and improve its clinical management is given in textbox 3. A major challenge is the high rate of misdiagnoses which can bias clinical trials towards non-inferiority.70 To determine applicability and reliability of trial results, it is imperative to document results from abscesses and cellulites separately, to accurately describe criteria and definitions, to extensively document clinical and microbiological characteristics, and to report information on additional procedures such as surgical drainage or limb immobilisation.134 For this relatively simple infection which has plagued humanity for so long, there still is a lot to discover.

ACKNOWLEDGEMENT
We thank Dr. Harjeet Singh Virk for critically reviewing the manuscript.
DISCLOSURES
The authors were supported by the Netherlands Organisation for Health Research and Development (ZonMW; grant no. 836011024). We have no conflicts of interest to report.
REFERENCES
- Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-35.
- Blackberg A, Trell K, Rasmussen M. Erysipelas, a large retrospective study of aetiology and clinical presentation. BMC Infect Dis. 2015;15:402.
- Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-55.
- Bruun T, Oppegaard O, Kittang BR, Mylvaganam H, Langeland N, Skrede S. Etiology of Cellulitis and Clinical Prediction of Streptococcal Disease: A Prospective Study. Open Forum Infect Dis. 2016;3:ofv181.
- Wielink G KS, Oosterhout RM, Wetzels R, Nijman FC, Draijer LW. NHG-Standaard Bacteriële huidinfecties (Eerste herziening). Huisarts Wet. 2007;50:426-44.
- Goettsch WG, Bouwes Bavinck JN, Herings RM. Burden of illness of bacterial cellulitis and erysipelas of the leg in the Netherlands. J Eur Acad Dermatol Venereol. 2006;20:834-9.
- Figtree M, Konecny P, Jennings Z, Goh C, Krilis SA, Miyakis S. Risk stratification and outcome of cellulitis admitted to hospital. J Infect. 2010;60:431-9.
- Lengte en gewicht van personen, ondergewicht en overgewicht; vanaf 1981 [Internet]. CBS Statline. 2016 [cited 05-10-2016]. Available from: http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=81565NED.
- Personen naar door de huisarts geregistreerde diagnose [Internet]. CBS Statline. 2016 [cited 05-10-2016]. Available from: http://statline.cbs. nl/Statweb/publication/?VW=T&DM=SLNL&PA=80193NED&D1=137&D 2=0&D3=0&D4=0&D5=a&HD=141111-1359&HDR=G3,G1,G2&STB=T,G4.
- Bevolking; kerncijfers [Internet]. CBS Statline. 2016 [cited 05-10-2016]. Available from: http://statline.cbs.nl/Statweb/publication/?DM=SLNL& PA=37296ned&D1=0-51&D2=0,10,20,30,40,50,(l-1)-l&VW=T.
- Lavrijsen APM DR, van Dissel JT, Draijer LW, et al. Richtlijn cellulitis en erysipelas van de onderste extremiteiten: Nederlandse Vereniging voor Dermatologie en Venereologie; 2013 [Available from: http://www.nvdv.nl/ wp-content/uploads/2014/08/Richtlijn-erysipelas-en-cellulitis-2013.pdf.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52.
- Chi YW, Raffetto JD. Venous leg ulceration pathophysiology and evidence based treatment. Vasc Med. 2015;20:168-81.
- Qaseem A, McLean RM, Starkey M, Forciea MA. Clinical Guidelines Committee of the American College of P. Diagnosis of Acute Gout: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:52-7.
- Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14:210-4.
- Sunderkotter C, Becker K. Frequent bacterial skin and soft tissue infections: diagnostic signs and treatment. J Dtsch Dermatol Ges. 2015;13:501-24; quiz 25-6.
- Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51:344-62.
- Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated With Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016. Nov 2. doi: 10.1001/jamadermatol.2016.3816. [Epub ahead of print].
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-8.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: A multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-5.
- Krasagakis K, Valachis A, Maniatakis P, Kruger-Krasagakis S, Samonis G, Tosca AD. Analysis of epidemiology, clinical features and management of erysipelas. Int J Dermatol. 2010;49:1012-7.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect. 2005;51:383-9.
- Bergan J. Molecular mechanisms in chronic venous insufficiency. Ann Vasc Surg. 2007;21:260-6.
- Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ. 2006;332:347-50.
- Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134:846-50.
- Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14:210-4.
- Snyder CD, Hitt NP, Young JA. Accounting for groundwater in stream fish thermal habitat responses to climate change. Ecol Appl. 2015;25:1397-419.
- Shin SU, Lee W, Park EA, Shin CI, Chung JW, Park JH. Comparison of characteristic CT findings of lymphedema, cellulitis, and generalized edema in lower leg swelling. Int J Cardiovasc Imaging. 2013;29 Suppl 2:135-43.
- Hietbrink F, Bode LG, Riddez L, Leenen LP, van Dijk MR. Triple diagnostics for early detection of ambivalent necrotizing fasciitis. World J Emerg Surg. 2016;11:51.
- Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518-27.
- Gaspari RJ, Resop D, Mendoza M, Kang T, Blehar D. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillinresistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-91 e1.
- Daum RS ML, Immergluck L, Fritz S, et al. Clindamycin Versus Trimethoprim-Sulfamethoxazole Versus Placebo for Uncomplicated Skin and Soft Tissue Abscesses. Open Forum Infect Dis. 2016;2016(3 (suppl_1)):1684.
- Talan DA, Mower WR, Krishnadasan A, et al. TrimethoprimSulfamethoxazole versus Placebo for Uncomplicated Skin Abscess. N Engl J Med. 2016;374:823-32.
- Bjornsdottir S, Gottfredsson M, Thorisdottir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-22.
- Kish TD, Chang MH, Fung HB. Treatment of skin and soft tissue infections in the elderly: A review. Am J Geriatr Pharmacother. 2010;8:485-513.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-16; quiz 17-20.
- Pereira de Godoy JM, Galacini Massari P, Yoshino Rosinha M, Marinelli Brandao R, Foroni Casas AL. Epidemiological data and comorbidities of 428 patients hospitalized with erysipelas. Angiology. 2010;61:492-4.
- Jorup-Ronstrom C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-6.
- Karppelin M, Siljander T, Vuopio-Varkila J, et al. Factors predisposing to acute and recurrent bacterial non-necrotizing cellulitis in hospitalized patients: a prospective case-control study. Clin Microbiol Infect. 2010;16:729-34.
- Compton GA. Bacterial skin and soft tissue infections in older adults. Clin Geriatr Med. 2013;29:443-59.
- Percival SL, Emanuel C, Cutting KF, Williams DW. Microbiology of the skin and the role of biofilms in infection. Int Wound J. 2012;9:14-32.
- Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-80.
- Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67-71.
- Rebell G, Pillsbury DM, De Saint Phalle M, Ginsburg D. Factors affecting the rapid disappearance of bacteria placed on the normal skin. J Invest Dermatol. 1950;14:247-64.
- Dupuy A, Benchikhi H, Roujeau JC, Bernard P, Vaillant L, Chosidow O, et al. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ. 1999;318:1591-4.
- Htwe TH, Mushtaq A, Robinson SB, Rosher RB, Khardori N. Infection in the elderly. Infect Dis Clin North Am. 2007;21:711-43, ix.
- Listi F, Candore G, Modica MA, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci. 2006;1089:487-95.
- Liang SY, Mackowiak PA. Infections in the elderly. Clin Geriatr Med. 2007;23:441-56, viii.
- Halpern J, Holder R, Langford NJ. Ethnicity and other risk factors for acute lower limb cellulitis: a U.K.-based prospective case-control study. Br J Dermatol. 2008;158:1288-92.
- Pugliese A, Vidotto V, Beltramo T, Torre D. Phagocytic activity in human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 2005;12:889-95.
- Stappers MH, Oosting M, Ioana M, et al. Genetic Variation in TLR10, an Inhibitory Toll-Like Receptor, Influences Susceptibility to Complicated Skin and Skin Structure Infections. J Infect Dis. 2015;212:1491-9.
- Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis. 2012;31:379-88.
- Suaya JA, Eisenberg DF, Fang C, Miller LG. Skin and soft tissue infections and associated complications among commercially insured patients aged 0-64 years with and without diabetes in the U.S. PLoS One. 2013;8:e60057.
- Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, Weigelt JA. Skin and soft tissue infections in hospitalised patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia. 2010;53:914-23.
- Olafsdottir LB, Erlendsdottir H, Melo-Cristino J, et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Euro Surveill. 2014;19:5-14.
- Oppegaard O, Mylvaganam H, Kittang BR. Beta-haemolytic group A, C and G streptococcal infections in Western Norway: a 15-year retrospective survey. Clin Microbiol Infect. 2015;21:171-8.
- SanMiguel A, Grice EA. Interactions between host factors and the skin microbiome. Cell Mol Life Sci. 2015;72:1499-515.
- Horton JM, Gao Z, Sullivan DM, Shopsin B, Perez-Perez GI, Blaser MJ. The cutaneous microbiome in outpatients presenting with acute skin abscesses. J Infect Dis. 2015;211:1895-904.
- Christensen GJ, Bruggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5:201-15.
- Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52 Suppl 1:i3-17.
- Marwick C, Broomhall J, McCowan C, et al. Severity assessment of skin and soft tissue infections: cohort study of management and outcomes for hospitalized patients. J Antimicrob Chemother. 2011;66:387-97.
- Marwick C, Rae N, Irvine N, Davey P. Prospective study of severity assessment and management of acute medical admissions with skin and soft tissue infection. J Antimicrob Chemother. 2012;67:1016-9.
- Hashem NG, Hidayat L, Berkowitz L, Venugopalan V. Management of skin and soft-tissue infections at a community teaching hospital using a severity-of-illness tool. J Antimicrob Chemother. 2016;71:3268-75.
- Lane S, Johnston K, Sulham KA, et al. Identification of Patient Characteristics Influencing Setting of Care Decisions for Patients With Acute Bacterial Skin and Skin Structure Infections: Results of a Discrete Choice Experiment. Clin Ther. 2016;38:531-44; quiz 44 e1-9.
- Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-31.
- Bader MS, Twells L, Hawboldt J. Risk factors of cellulitis treatment failure with once-daily intravenous cefazolin plus oral probenecid. South Med J. 2011;104:789-93.
- Volz KA, Canham L, Kaplan E, Sanchez LD, Shapiro NI, Grossman SA. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-4.
- Sabbaj A, Jensen B, Browning MA, John Ma O, Newgard CD. Soft tissue infections and emergency department disposition: predicting the need for inpatient admission. Acad Emerg Med. 2009;16:1290-7.
- Corwin P, Toop L, McGeoch G, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ. 2005;330:129.
- Obaitan I, Dwyer R, Lipworth AD, et al. Failure of antibiotics in cellulitis trials: a systematic review and meta-analysis. Am J Emerg Med. 2016;34:1645-52.
- Lipsky BA, Napolitano LM, Moran GJ, et al. Economic outcomes of inappropriate initial antibiotic treatment for complicated skin and soft tissue infections: a multicenter prospective observational study. Diagn Microbiol Infect Dis. 2014;79:266-72.
- Bauer S, Aubert CE, Richli M, Chuard C. Blood cultures in the evaluation of uncomplicated cellulitis. Eur J Intern Med. 2016;36:50-6.
- Lee CY, Kunin CM, Chang C, Lee SS, Chen YS, Tsai HC. Development of a prediction model for bacteremia in hospitalized adults with cellulitis to aid in the efficient use of blood cultures: a retrospective cohort study. BMC Infect Dis. 2016;16:581.
- Paolo WF, Poreda AR, Grant W, Scordino D, Wojcik S. Blood culture results do not affect treatment in complicated cellulitis. J Emerg Med. 2013;45:163-7.
- Drinka P, Bonham P, Crnich CJ. Swab culture of purulent skin infection to detect infection or colonization with antibiotic-resistant bacteria. J Am Med Dir Assoc. 2012;13:75-9.
- Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-7.
- Piso RJ, Pop R, Wieland M, et al. Low sensitivity of needle aspiration cultures in patients with cellulitis/erysipelas. Springerplus. 2016;5:1578.
- Lee CY, Tsai HC, Kunin CM, Lee SS, Chen YS. Clinical and microbiological characteristics of purulent and non-purulent cellulitis in hospitalized Taiwanese adults in the era of community-associated methicillin-resistant Staphylococcus aureus. BMC Infect Dis. 2015;15:311.
- Karppelin M, Siljander T, Haapala AM, et al. Evidence of streptococcal origin of acute non-necrotising cellulitis: a serological study. Eur J Clin Microbiol Infect Dis. 2015;34:669-72.
- Mebis J, Jansens H, Minalu G, et al. Long-term epidemiology of bacterial susceptibility profiles in adults suffering from febrile neutropenia with hematologic malignancy after antibiotic change. Infect Drug Resist. 2010;3:53-61.
- Sood A, Midha V, Goyal O, et al. Skin and soft tissue infections in cirrhotics: a prospective analysis of clinical presentation and factors affecting outcome. Indian J Gastroenterol. 2014;33:281-4.
- Mohan P, Ramu B, Bhaskar E, Venkataraman J. Prevalence and risk factors for bacterial skin infection and mortality in cirrhosis. Ann Hepatol. 2011;10:15-20.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885-910.
- Diaz JH, Lopez FA. Skin, soft tissue and systemic bacterial infections following aquatic injuries and exposures. Am J Med Sci. 2015;349:269-75.
- Imberg R, Potasman I, Weissman Y, Grupper M. Hand infections following penetrating fish fins or bones injuries (FFBI): a large, hospital based, retrospective study. Infection. 2008;36:565-9.
- Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
- Jenkins TC, Knepper BC, Jason Moore S, et al. Comparison of the microbiology and antibiotic treatment among diabetic and nondiabetic patients hospitalized for cellulitis or cutaneous abscess. J Hosp Med. 2014;9:788-94.
- Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008;61:235-7.
- Saeed K, Marsh P, Ahmad N. Cryptic resistance in Staphylococcus aureus: a risk for the treatment of skin infection? Curr Opin Infect Dis. 2014;27:130-6.
- NethMap 2016: Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands / MARAN 2016: Monitoring of Antimicrobial Resistance of Antibiotic Usage in Animals in the Netherlands in 2015 [Internet]. National Institute for Public Health and the Environment. 2016. Available from: http://www. rivm.nl/dsresource?objectid=752059cb-4dfa-42ec-a013-60bc21e52508&t ype=org&disposition=inline.
- Kilburn SA, Featherstone P, Higgins B, Brindle R. Interventions for cellulitis and erysipelas. Cochrane Database Syst Rev. 2010:CD004299.
- Bernard P, Chosidow O, Vaillant L, French Erysipelas Study G. Oral pristinamycin versus standard penicillin regimen to treat erysipelas in adults: randomised, non-inferiority, open trial. BMJ. 2002;325:864.
- Madaras-Kelly KJ, Remington RE, Oliphant CM, Sloan KL, Bearden DT. Efficacy of oral beta-lactam versus non-beta-lactam treatment of uncomplicated cellulitis. Am J Med. 2008;121:419-25.
- Ferreira A, Bolland MJ, Thomas MG. Meta-analysis of randomised trials comparing a penicillin or cephalosporin with a macrolide or lincosamide in the treatment of cellulitis or erysipelas. Infection. 2016;44:607-15.
- Tsoulas C, Nathwani D. Review of meta-analyses of vancomycin compared with new treatments for Gram-positive skin and soft-tissue infections: Are we any clearer? Int J Antimicrob Agents. 2015;46:1-7.
- Deak D, Outterson K, Powers JH, Kesselheim AS. Progress in the Fight Against Multidrug-Resistant Bacteria? A Review of U.S. Food and Drug Administration-Approved Antibiotics, 2010-2015. Ann Intern Med. 2016;165:363-72.
- Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:2169-79.
- Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60:254-62.
- Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:2180-90.
- Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309:559-69.
- Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14:696-705.
- Shorr AF, Lodise TP, Corey GR, et al. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2015;59:864-71.
- Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A Randomized Clinical Trial of Single-Dose Versus Weekly Dalbavancin for Treatment of Acute Bacterial Skin and Skin Structure Infection. Clin Infect Dis. 2016;62:545-51.
- Farmacotherapeutisch Kompas [Internet]. Zorginstituut Nederland. [cited 05-10-2016]. Available from: http://www.farmacotherapeutischkompas.nl/.
- Tucker CE, Lockwood AM, Nguyen NH. Antibiotic dosing in obesity: the search for optimum dosing strategies. Clin Obes. 2014;4:287-95.
- Theofiles M, Maxson J, Herges L, Marcelin A, Angstman KB. Cellulitis in Obesity: Adverse Outcomes Affected by Increases in Body Mass Index. J Prim Care Community Health. 2015;6:233-8.
- Halilovic J, Heintz BH, Brown J. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65:128-34.
- Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. 2004;164:1669-74.
- Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-90.
- Uranga A, Espana PP, Bilbao A, et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multicenter Randomized Clinical Trial. JAMA Intern Med. 2016;176:1257-65.
- Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372:1996-2005.
- Cranendonk DR, Opmeer BC, Prins JM, Wiersinga WJ. Comparing short to standard duration of antibiotic therapy for patients hospitalized with cellulitis (DANCE): study protocol for a randomized controlled trial. BMC Infect Dis. 2014;14:235.
- Pitche PV, Saka B, Diatta AB, et al. Risk factors associated with abscess formation among patient with leg erysipelas (cellulitis) in sub-Saharan Africa: a multicenter study. BMC Dermatol. 2015;15:18.
- Krasagakis K, Samonis G, Valachis A, Maniatakis P, Evangelou G, Tosca A. Local complications of erysipelas: a study of associated risk factors. Clin Exp Dermatol. 2011;36:351-4.
- Reich-Schupke S, Murmann F, Altmeyer P, Stucker M. Quality of life and patients’ view of compression therapy. Int Angiol. 2009;28:385-93.
- Edwards LM. Why patients do not comply with compression bandaging. Br J Nurs. 2003;12(11 Suppl):S5-6, S8, S10 passim.
- Beldon P. Compression bandaging: avoiding pressure damage. Br J Community Nurs. 2008;13:S6, S8, S10-2.
- Stalbow J. Preventing cellulitis in older people with persistent lower limb oedema. Br J Nurs. 2004;13:725-32.
- Dall L, Peterson S, Simmons T, Dall A. Rapid resolution of cellulitis in patients managed with combination antibiotic and anti-inflammatory therapy. Cutis. 2005;75:177-80.
- Bergkvist PI, Sjobeck K. Antibiotic and prednisolone therapy of erysipelas: a randomized, double blind, placebo-controlled study. Scand J Infect Dis. 1997;29:377-82.
- Bergkvist PI, Sjobeck K. Relapse of erysipelas following treatment with prednisolone or placebo in addition to antibiotics: a 1-year follow-up. Scand J Infect Dis. 1998;30:206-7.
- Inghammar M, Rasmussen M, Linder A. Recurrent erysipelas--risk factors and clinical presentation. BMC Infect Dis. 2014;14:270.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-15.
- Cox NH. Oedema as a risk factor for multiple episodes of cellulitis/ erysipelas of the lower leg: a series with community follow-up. Br J Dermatol. 2006;155:947-50.
- Karppelin M, Siljander T, Aittoniemi J, et al. Predictors of recurrent cellulitis in five years. Clinical risk factors and the role of PTX3 and CRP. J Infect. 2015;70:467-73.
- Hemmige V, McNulty M, Silverman E, David MZ. Recurrent skin and soft tissue infections in HIV-infected patients during a 5-year period: incidence and risk factors in a retrospective cohort study. BMC Infect Dis. 2015;15:455.
- Brodell LA, Brodell JD, Brodell RT. Recurrent lymphangitic cellulitis syndrome: A quintessential example of an immunocompromised district. Clin Dermatol. 2014;32:621-7.
- O’Neill AM, Thurston TL, Holden DW. Cytosolic Replication of Group A Streptococcus in Human Macrophages. MBio. 2016;7:e00020-16.
- Blaszczyk-Kostanecka M, Dobozy A, Dominguez-Soto L, et al. Comparison of two regimens of oral clindamycin versus dicloxacillin in the treatment of mild-to-moderate skin and soft-tissue infections. Curr Ther Res Clin Exp. 1998;59:341-53.
- Oh CC, Ko HC, Lee HY, Safdar N, Maki DG, Chlebicki MP. Antibiotic prophylaxis for preventing recurrent cellulitis: a systematic review and meta-analysis. J Infect. 2014;69:26-34.
- Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013;368:1695-703.
- Ellis MW, Schlett CD, Millar EV, et al. Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: a cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis. 2014;58:1540-8.
- Weintrob A, Bebu I, Agan B, et al. Randomized, Double-Blind, Placebo-Controlled Study on Decolonization Procedures for MethicillinResistant Staphylococcus aureus (MRSA) among HIV-Infected Adults. PLoS One. 2015;10:e0128071.
- McClaine RJ, Husted TL, Hebbeler-Clark RS, Solomkin JS. Meta-analysis of trials evaluating parenteral antimicrobial therapy for skin and soft tissue infections. Clin Infect Dis. 2010;50:1120-6.

