

KEYWORDS
IgA nephropathy, immunosuppression, therapy, end-stage renal disease, progression
INTRODUCTION
IgA nephropathy is the most common glomerular disease worldwide. Its natural course is highly variable and far from benign in many patients.1 Overall, approximately 25% of patients experience a lasting remission,2 whereas 30% of patients develop end-stage renal disease (ESRD) within 20 years.3 Risk factors associated with disease progression include heavy proteinuria, impaired baseline renal function and renal morphological lesions such as the presence of segmental sclerosis and the degree of tubular atrophy/interstitial fibrosis.4-12
Many patients with IgA nephropathy can be effectively treated with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) (The Kidney Disease: Improving Global Outcomes (KDIGO) Guideline 2012). There is evidence to support the use of corticosteroids in patients with persistent proteinuria and minimal renal impairment.13-16 It is debated whether patients with IgA nephropathy and renal insufficiency benefit from more aggressive treatment consisting of corticosteroids combined with alkylating agents. The evidence is derived from small studies, mostly conducted in a period with less defined blood pressure targets and variable use of ACEi/ARB as opposed to the current guidelines.17-20 More specifically, evidence favouring the use of cyclophosphamide in patients with non-crescentic IgA nephropathy and severe renal insufficiency is derived from a single small controlled trial.18 During the last decade we have used cytotoxic therapy including cyclophosphamide in patients with advanced or severe IgA nephropathy. In the present study, we aimed to determine the response to immunosuppressive therapy in patients with progressive IgA nephropathy. Specifically, we sought to identify possible predictors of response.
METHODS
Patients
We retrospectively analysed data of adults with biopsy-proven IgA nephropathy who were referred for therapeutic advice to the Radboud University Medical Centre and the Leiden University Medical Centre and were subsequently treated with cytotoxic agents because of progressive IgA nephropathy. Patients with evidence of systemic disease, such as systemic lupus erythematosus, chronic liver disease and Henoch-Schönlein purpura or a follow-up of less than 12 months were excluded. Relevant clinical and biochemical data were retrieved from patient records. Baseline was defined as the date of first presentation. During follow-up quantitative analysis of proteinuria was assessed by the ratio of urinary protein to urinary creatinine in the majority of patients. This parameter is closely correlated with 24-hour urinary protein excretion. This study was carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent.
Treatment protocol
Standard immunosuppressive therapy for IgA nephropathy consisted of prednisone 40 mg per day with monthly tapering for a maximum period of 24 months plus cyclophosphamide (1.5 mg/kg) during the first three months orally followed by azathioprine (1.5 mg/kg) for 18 months (derived from study treatment protocol by Ballardie et al.). 18 During prednisone therapy, concomitant treatment with H2 -blockers or proton pump inhibitors was used. Co-trimoxazole was prescribed to prevent Pneumocystic jiroveci infections during the administration of cyclophosphamide. Young women were treated with azathioprine and not with cyclophosphamide because the latter is associated with infertility. Severe leukopenia, infections or active malignancy were regarded as stringent reasons to discontinue immunosuppressive treatment.
Assessment of response
The primary outcome measure was progression of renal disease defined as an increase in serum creatinine levels of ≥ 50% or development of ESRD (serum creatinine ≥ 500 µmol/l, dialysis or renal transplantation). Proteinuria responses were evaluated in terms of a reduction in proteinuria of 50% or a persistent urine protein-creatinine ratio < 1 g/10 mmol.
Calculations
The glomerular filtration rate was estimated (eGFR) by the simplified Modification of Diet in Renal Disease (MDRD) formula.21 Mean arterial pressure was calculated as the diastolic pressure plus one-third of the pulse pressure.
Statistical analysis
Data values are expressed as mean ± standard deviation or median (range) as appropriate. Parameters between groups were compared using the Mann-Whitney test for non-parametric continuous data, the independent t-test for parametric data, and the chi-square test for categorical data. A p-value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS for windows software, version 20 (IBM SPSS).
RESULTS
Baseline characteristics
Between 1996 and 2008, 19 patients received immunosuppressive therapy because of progressive IgA nephropathy. Standard immunosuppressive treatment consisting of prednisone plus cyclophosphamide during the first three months orally followed by a cytotoxic agent was prescribed in 11 out of 19 patients. Five patients received prednisone plus cyclophosphamide without subsequent alternative agent. This was due to side effects (leucopenia, infection) or failure of improvement of already severely impaired renal function (n = 1). Three patients were started on azathioprine plus prednisone instead of cyclophosphamide plus prednisone. Clinical characteristics at presentation are depicted in table 1.
At presentation, mean age was 39±11 years, median serum creatinine was 128 μmol/l, a median eGFR of 55 ml/ min/1.73 m2 , and median proteinuria was 4.5 g/10 mmol creatinine. All patients but one were treated with ACEI or ARB prior to the initiation of immunosuppressive therapy. Three patients had previously been treated with prednisone monotherapy. In the majority of patients therapy was started because of an increase in serum creatinine of 15% or more in the previous 12 months (n = 12), or because of a serum creatinine > 135 μmol/l and proteinuria > 1 gram per day (n = 6). In one patient therapy was initiated because of severe impairment of renal function at the time of presentation. The median time between baseline and start of therapy was 19 months. At start of therapy, patients were 42±11 years and had a median serum creatinine of 208 μmol/l, a median eGFR of 33 ml/min/1.73 m2 , and a median urine protein-creatinine ratio of 3.8 g/10 mmol (table 1).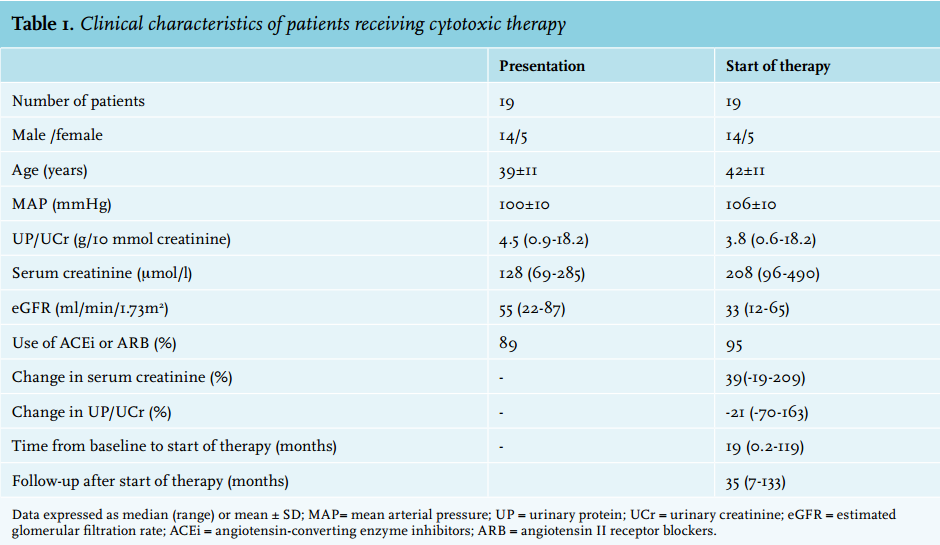
Follow-up after initiation of therapy was 35 (7-133) months. In ten patients renal disease was progressive despite the initiation of immunosuppressive therapy: six developed ESRD at a median follow-up of 24 (range 7-46) months and four experienced doubling of serum creatinine after 17 (range 12-81) months. Renal function remained stable or improved in nine patients.
Serum creatinine levels and proteinuria at the start of treatment were lower in responders (183 (120-381) versus 224 (96-490) μmol/l, p=0.23 and 2.2 (0.6-9.7) versus 4.1 (0.7-18.2) g/10 mmol creatinine, p = 0.31 respectively), but these differences were not statistically significant (table 2). There were no differences in other characteristics at baseline between responders and non-responders (table 2). Blood pressures were well controlled during treatment. Of note, there were no differences in mean arterial pressure between responders and non-responders (table 2). In eight out of nine responders proteinuria decreased by ≥ 50% within six months and attained levels below 1 g/day in eight patients (figure 1A). In contrast, in non-responders proteinuria decreased by more than 50% and reached values < 1g/day in only three out of ten patients (p < 0.01, figure 1B).
Treatment duration was 19 (range 3-81) months. Treatment duration was less than 12 months in two of the responders, while four of the non-responders received treatment for less than 12 months (table 3). Following the discontinuation of therapy, we observed no relapses of proteinuria.
Six patients had a serum creatinine level ≥ 250 µmol/l (the point of no return) prior to the start of therapy. In this small subgroup renal function remained stable after treatment in two patients during a follow-up of more than eight years. The remaining four patients developed ESRD within 21-46 months after initiation of treatment. We evaluated the possible predictive value of pathological characteristics. In many patients the interval between kidney biopsy and start of immunosuppressive therapy was > 12 months (n = 10). Only nine biopsies were classified using the Oxford MEST scores (median eGFR 20 (range 15-42) ml/min/1.73 m2 , median proteinuria 4.2 g/10 mmol creatinine (range 0.7-18) at start of therapy), in six of these patients time between renal biopsy and start of therapy was < 4 months, in the other three patients the interval between renal biopsy and start of therapy was > 20 months. Four out of these nine patients were non-responders to therapy. When comparing non-responders and responders, non-responders seemed to exhibit more mesangial hypercellularity and more tubulointerstitial fibrosis, variables associated with progression of renal disease. Surprisingly, non-responders also seemed to have more endocapillary proliferation, a variable that was not associated with a decline in renal function in recent validation studies of the Oxford score.
Adverse effects of treatment
Adverse events were reported in 11 patients (58%) and included anaemia (n = 3), leucopenia (n = 4), thrombocytopenia (n = 1), infection (n = 4) or liver toxicity (n = 1). In two patients this led to discontinuation of immunosuppressive therapy. None of the patients developed malignancy during follow-up.
DISCUSSION
This study suggests that immunosuppressive therapy may result in stabilisation of renal function in approximately 50% of patients with IgA nephropathy at high risk for progression of renal disease. More importantly, a decrease in proteinuria of 50% or more within six months predicted a sustained response to therapy.
Over two decades, beneficial effects of corticosteroids have been reported by various studies in patients with no or mild renal impairment.13,22-25 However, in patients with advanced IgA nephropathy, administration of corticosteroids did not improve renal function.26 A few randomised controlled trials, enrolling a small number of patients, have been conducted to investigate the efficacy of cytotoxic agents combined with prednisone.17-20,27 Only the study performed by Ballardie et al. showed that prednisone plus cytotoxic agents were effective in preserving renal function in patients with moderate, progressive disease.18 Thus, the optimal management of patients with advanced IgA nephropathy remains controversial.
Clearly, this small, retrospective and uncontrolled study cannot provide compelling evidence for benefit of corticosteroids and cyclophosphamide or azathioprine in high-risk IgA nephropathy patients. Yet, our data support the results reported by Ballardie; based on observational data on known major risk factors, at least 70-90% of this study population characterised by heavy, persistent proteinuria, (median 3.1 g/d) and severe impairment of renal function (median serum creatinine 208 µmol/l) at the start of treatment with immunosuppressive drugs, would be expected to progress to ESRD.28,29 However, after immunosuppressive therapy progression of disease was halted and renal function remained stable in approximately 50% of patients. Admittedly, responders to therapy had slightly better renal function and less heavy proteinuria at the start of therapy. However, clinical characteristics of the responders still predict a dismal outcome. Of note, there were no differences in blood pressures between the responder and non-responder groups (table 2).
Thus, immunosuppressive combination therapy may benefit IgA nephropathy patients with a poor prognosis. Unfortunately, side effects are frequently observed, duration of treatment is long and 50% of patients are exposed to cytotoxic agents and prednisone to no avail. Therefore, immunosuppressive therapy seems to benefit only a subset of high-risk patients with IgA nephropathy. Currently it is impossible to identify this subgroup by means of clinical or pathological characteristics. Considering this, our finding that a 50% decrease in proteinuria within six months after the start of therapy is indicative of a sustained response to immunosuppressive therapy is highly relevant. In patients lacking a substantial decrease in proteinuria after six months of therapy, discontinuation of treatment should be strongly considered, in our opinion.
Our study did not allow conclusions regarding the role of the histological classification to predict response to immunosuppressive therapy because the numbers were too small. Of note, this question was addressed in a recent European study intended to validate the Oxford classification.30 In this study, the predictive value of the pathology score was no longer present in the patients who received immunosuppressive therapy.
Recently, it has been recognised that patients with IgA nephropathy have increased serum levels of galactosedeficient IgA1 and that circulating autoantibodies recognising this galactose-deficient IgA1 as an autoantigen or the levels of the autoantigen itself allow prediction of disease progression.28,31 Perhaps these and other promising biomarkers will allow identification of patients responsive to immunosuppressive therapy in the near future.
The results of this retrospective study differ to some extent from the data reported by Ballardie et al. A substantially larger number of patients suffered from therapy-related side effects in our study compared with the frequency reported by Ballardie and others (58% vs 7-12%).18,32 Although these side effects were mild and transient in the majority of cases, two patients withdrew from treatment. Second, several studies including Ballardie’s reported that patients with a serum creatinine level ≥ 250 µmol/l inescapably develop ESRD despite therapy.18,33,34 We observed a sustained stabilisation of renal function in two out of six patients beyond this point of no return after therapy. Thus, even in patients with severe renal impairment, immunosuppressive therapy may be of benefit.
In conclusion, the optimal management of IgA nephropathy remains controversial and randomised controlled trials are much needed. Currently, a large randomised prospective controlled trial (STOP IgAN trial) is being conducted in order to determine whether immunosuppressive therapy is able to prevent progression of renal disease.35 The KDIGO Clinical Practice Guideline for Glomerulonephritis suggests corticosteroid therapy in patients with persistent proteinuria > 1 g/d despite 3-6 months of optimised supportive care (including ACEi/ ARB) and eGFR > 50 ml/min. Combined immunosuppressive therapy is not advised unless there is crescentic IgA nephropathy with rapidly deteriorating renal function. In our opinion, immunosuppressive therapy can certainly be considered in IgA nephropathy patients with a decline in renal function and persistent proteinuria > 1 g/d. Immunosuppressive therapy should only be continued beyond six months in those patients demonstrating a significant (> 50%) decrease in proteinuria.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES