

KEY WORDS
Hepatitis B reactivation, immunosuppressive therapy, screening
INTRODUCTION
Infection with hepatitis B virus (HBV) is a global public health problem that leads to significant morbidity and mortality. According to the World Health Organization (WHO), an estimated 240 million people are chronically infected with hepatitis B worldwide.1 The regional endemic variation of hepatitis B infected patients is large, with currently changing prevalence and incidence in low endemic countries as a result of the higher hepatitis B prevalence in migrants and refugees.2-4 According to the Pienter studies, prevalence in the Netherlands in 2007 was 0.2% for a chronic HBV infection (positive hepatitis B surface antigen, HBsAg), and 3.5% for patients ever exposed to HBV (antibodies to hepatitis B core antigen, anti-HBc).5 A significantly higher prevalence was observed within the population of non-Western immigrants and high-risk groups. Both patients with a chronic HBV infection and patients exposed to HBV (with or without antibodies to HBsAg (anti-HBs)) are at risk for reactivation. Reactivation is generally defined as a detectable HBV-DNA level in patients with a previously undetectable HBV-DNA level, a significant increase of HBV-DNA (> 10 fold or > 2,000 IU/ml) and/or the reappearance of HBsAg in a previously negative individual.6 Known risk factors for reactivation are immuno- or chemotherapy, organ transplantation and other infectious diseases such as HIV and hepatitis C. A considerable number of patients are unaware of their HBV status and the risk factors for HBV infection.7,8
In particular, patients treated with B-cell depleting agents such as rituximab or combined immunosuppressive therapy containing steroids are at risk for reactivation of HBV (HBVr).9-11 Reactivation can cause a fulminant infection that can lead to severe liver failure and even death, with a mortality rate of up to 25%.9 Furthermore, chemotherapy treatment may be interrupted or delayed in cases of HBVr.
Selective screening for at risk patients is ineffective and high-risk patients are not always correctly assessed or recognized.9 Therefore, current international guidelines recommend a routine HBV screening procedure for every patient planned to receive cancer treatment.2,10,12,13 Recently, a Dutch HBV guideline based on the guidelines of the European Association of the Study of the Liver (EASL) was published and states that all patients must be screened for HBV before the start of chemotherapy.14 Despite the realization that routine screening for both solid and hematological malignancies is needed, the actual screening rate by oncologists remains low, 13-19%.15,16 Oncologists who experienced HBVr in a patient were more likely to screen all patients compared to oncologists who did not.16
In our hospital, a routine HBV screening protocol was introduced for every patient initiated for any type of chemotherapy in September 2015, after two cases of HBVr occured. The first case was a North-African woman treated with a rituximab-containing regimen; this patient died as a consequence of severe liver failure. The other patient experienced transient liver failure after treatment with doxorubicin, cyclophosphamide and paclitaxel.17 Before routine HBV screening, only high-risk patients were screened, mainly based on ethnicity or type of immunosuppressive therapy.
In this retrospective study we report the incidence of a positive test result at screening for hepatitis B, defined as HBsAg positive and/or anti-HBc positive, in patients who started chemotherapy in our hospital. Furthermore, we will evaluate the introduction of routine HBV screening in all patients.
MATERIALS AND METHODS
Study population
A retrospective study was performed to investigate the incidence of a positive test result at screening for HBV in a patient population receiving chemotherapy. This study was carried out in the Medical Centre Slotervaart Amsterdam, a teaching hospital, in the internal medicine department. All patients who started with intravenous chemotherapy between January 2015 and October 2016 were included. This study was approved by the accredited Medical Ethics Committee of the hospital.
Hepatitis B screening
Before September 2015, patients were screened for HBV if they were considered high-risk patients by the treating physician. From that date on, a protocol for routine screening was introduced. A meeting was organized to inform all involved parties about the protocol and the protocol was published on the hospital’s document management system to be easily accessible for all healthcare providers within the hospital. We defined two different groups of patients: patients before the introduction of the protocol for HBV screening and patients after introduction of the protocol for HBV screening.
The standard HBV screening consists of HBsAg, anti-HBc and anti-HBs. If either the HBsAg and/or anti-HBc were positive, an HBV-DNA test was ordered. We used the Liaison XL test (DiaSorin, Sallugia, Italy) for hepatitis B serology, and the Abbott m2000 sp/rt system (Abbott Laboratories) for viral load (HBV DNA). The cut-off values were: HBsAg in IU/ml: < 0.05 was negative and > 0.05 was positive; anti-HBs in mIU/ml: < 9 was negative, ≥ 9 and ≤ 10 was equivalent and > 10 was positive. For anti-HBc, a qualitative test was performed with a positive or negative test result. HBV-DNA ≤ 10 IU/ml was considered negative. If a patient tested positive for anti-HBc, a standard follow-up with liver serum transaminases and three, monthly HBV-DNA tests were performed. Antiviral prophylactic therapy with tenofovir was started in patients with a positive HBsAg test or a positive HBV-DNA test, and in patients who were anti-HBc positive and started high-risk therapy.
Data collection
Patients who received their first chemotherapy treatment between January 2015 and October 2016 were identified through a database of the oncology department. The data were collected through the electronically registered medical records. Test results of HBV screening were recorded for every patient as well as patient characteristics including age, sex, nationality and type of cancer.
Two groups were defined based on the endemic appearance of hepatitis B in the patients’ country of birth: low endemic regions (< 2%) and high endemic regions (> 2%). Countries with a prevalence of chronic HBV infection (< 2%) were considered as low endemic (Western Europe and North America). Countries with a prevalence of chronic HBV infection of > 2% were considered as high endemic (Eastern Europe, Asia, Africa, South America, Middle East and the Caribbean).
Data analysis
Descriptive statistics were used to give an overview of the patient characteristics in this study. Categorical data were reported as numbers and percentages. Continuous data such as age were reported as median and interquartile range (IQR). To compare the isolated anti-HBc positive patients with the anti-HBc and anti-HBs positive patients we used the independent t-test for the continuous normal distributed variables and the Chi-Square test or Fisher’s exact test for the categorical variables, depending on the number of cases. All analyses were performed with IBM SPSS statistics (version 24).
RESULTS
Patient characteristics
In total, we included 184 patients with a solid or hematological malignancy for which they received their first treatment with intravenous chemotherapy at the Medical Centre Slotervaart between January 2015 and October 2016. Routine HBV screening for all patients was introduced in September 2015. The patients were divided in two groups: patients who started treatment before introduction of the screening protocol (group ‘before’) and the patients who started treatment after introduction of the screening protocol (group ‘after’) (table 1).
Screening procedure
Overall, 129 patients were screened for HBV according to the local screening procedure, 70 in the group ‘before’ and 114 in the group ‘after’. In the group ‘before’, 52.9% (37/70) of the patients were screened, and in the group ‘after’, 80.7% (92/114) of the patients were screened. The median age in both groups was approximately 60 years. In both groups, 64% of the patients were female. The most common malignancies were breast cancer, lung cancer, hematological cancer and colorectal cancer. Three-quarters of the patients were born in a low endemic country and 22% of the patients were born in a high endemic region.
Before introduction of the protocol for routine screening, the overall percentage of patients screened for HBV was 52.9%. Almost all hematological patients were screened (91%), while the HBV screening rate of patients with solid tumors ranged from 22-58% (table 1).
After introduction of the protocol in September 2015, the percentage of patients that were actually screened increased to 80.7%. The screening rate in patients with solid tumors increased from 22-58% to 42-98%. The screening rate in patients born in non-endemic areas increased from 48% in the group ‘before’ to 79% in the group ‘after’. Patients with a lung tumor were screened in 42% of the group ‘after’ cases; this was the lowest screening rate reported in the group after introduction of the protocol. The hematological patient group was the only group with a decrease in screening rate, from 91% to 80%.
Serology results
None of the 129 patients screened for HBV were HBsAg positive. Overall, 21 (16.3%) patients were anti-HBc positive and were therefore considered to be at risk for HBVr (table 2). In the group ‘before’, 8/37 (21.6%) of the screened patients tested positive for anti-HBc, of which five patients were both anti-HBc and anti-HBs positive and three patients were only anti-HBc positive (isolated anti-HBc). All of these patients tested negative for HBV DNA (HBV-DNA < 10 IU/ml). One of the three patients with an isolated anti-HBc started prophylactic antiviral therapy because of treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP).
In the group ‘after’, 13/92 (14.1%) of the screened patients tested positive for anti-HBc of which eight patients were both anti-HBc and anti-HBs positive and five patients were isolated anti-HBc positive. One of these five patients had HBV DNA levels > 10 IU/ml and started with prophylactic tenofovir before the start of chemotherapy. In this group, nine patients (7%) were isolated anti-HBs positive, most likely indicating a post-vaccination status.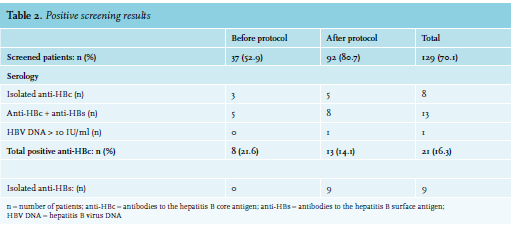
Because of the known risk of reactivation in patients with a positive anti-HBc, we will highlight this specific subgroup of 21 patients. The median age of these patients was 60 years old. Seven of these patients were born in a non-endemic region. Eight patients tested isolated anti-HBc positive, and five of these patients were born in a non-endemic area and three in an endemic area.
The age of patients with an isolated anti-HBc was significantly higher compared to patients with both a positive anti-HBc and anti-HBs, respectively 73 years and 53 years (p-value < 0.01). No significant difference was found for endemic background between patients with an isolated anti-HBc and patients with both a positive anti-HBc and anti-HBs.
Within the total anti-HBc positive group of patients, one patient had a history of a recovered HBV infection and one patient was known to have a concomitant HIV infection.
Reactivation and one-year follow-up
Within this study cohort, one patient suffered from an HBVr in the group before introduction of the routine HBV screening protocol. This patient was not screened before the start of the chemotherapy treatment for breast cancer with 4x doxorubicin/cyclophosphamide and 12x paclitaxel. The reactivation occurred one month after the last chemotherapy. From a stored blood sample, it was determined that the anti-HBc and anti-HBs (112 mIU/ml) were positive before start of the chemotherapy. During reactivation, the HBV DNA viral load reached almost 1,000,000 IU/ml and the anti-HBs test was negative. This patient was immediately started on tenofovir 245mg once daily for a period of 12 months and she fully recovered with a re-seroconversion to anti-HBs.17
A one-year follow-up was performed in the 21 patients with a positive anti-HBc test. In this group, two patients started tenofovir prophylaxis. The first patient was prone for reactivation because of a positive anti-HBc, a negative anti-HBs and a detectable HBV-DNA viral load between 10 IU/ml and 15 IU/ml. This patient was prescribed tenofovir 245 mg once daily at the start of chemotherapy untill 12 months after the last chemotherapy (R-miniCHOP). The other patient who received tenofovir was initiated for the high-risk R-CHOP chemotherapy and tested isolated anti-HBc positive with an HBV-DNA viral load < 10 IU/ml. The HBV-DNA viral load was undetectable during follow-up after start of chemotherapy in all other patients.
DISCUSSION
In the present study, we evaluated the introduction of a protocol for routine HBV screening in patients before start of medical cancer treatment in our hospital. Introduction of the screening protocol did not guarantee that all patients were screened. After introduction of the protocol, the screening rate increased from approximately 50% to 80%, but did not reach the desired 100% screening rate. This suboptimal screening rate can be partly explained by the low screening rate of patients with lung tumors (42%) compared to patients with other solid tumors or hematological malignancies (80-98%). Dutch treatment guidelines for lung tumors do not mention hepatitis B screening before start of therapy, although HBVr during lung cancer chemotherapy has been described.18 In recent years, national and international guidelines have started to recommend routine HBV screening before start of chemotherapy.2,12-14 However, these guidelines were written by international liver associations and have been published in their official journals. We believe that attention for HBV screening in oncology journals and/or implementation in oncology guidelines is needed to increase awareness amongst oncologists, as Van Roon et al. did in The Dutch Journal for Oncology. 19
Another observation was the decreased screening rate in patients with a hematological malignancy. Although the screening protocol was introduced, two patients received high-risk chemotherapy without HBV screening. In order to achieve a 100% screening rate, we organized a meeting to notify the involved doctors and oncology nurses about our findings and to discuss possible improvements for the HBV screening protocol. We have strongly advised to include HBV screening as a mandatory field on the checklist for every oncology patient. In addition, an automated warning system to check HBV status in the electronic patient record before chemotherapy prescription will help improve results.
A remarkable result of this study was the high prevalence (14%) of anti-HBc positive patients within the screened patients after introduction of the protocol. This is much higher compared to the incidence in the general Dutch population (3.5% in 2007).5 The fact that our hospital is located in a multi-ethnic area with many first- and second-generation immigrants may be a possible explanation. Another Dutch study performed in a multi-ethnic neighborhood of Rotterdam reported an equivalent prevalence of positive anti-HBc of 16%, weighted by sex and ethnicity.20 Interestingly, one-third of the patients with a positive anti-HBc was born in a low-endemic country. This illustrates that selective screening of high-risk patients for HBV is not effective.7,9 It should be noted however, that of the 21 patients with a positive HBV test result, eight were isolated anti-HBc positive. Of these patients, five were born in a non-endemic region. This raises the question whether these patients had a previous HBV infection with loss of measurable anti-HBs or a false-positive anti-HBc test result. Multiple diagnostic tests are available for testing anti-HBc and these different tests can show various outcomes within the same patient.21 According to a large Dutch study in donor patients, low-reactivity of an anti-HBc test in combination with undetectable anti-HBs and a Western European background suggests false positivity.22 In contrast, an isolated positive anti-HBc test in combination with a patient born in an endemic country (> 2%) is a reason to assume a true test result and requires further action.20,22 As a result it is possible that the prevalence of patients with a history of HBV in our study cohort is overestimated. Interestingly, the age of patients with an isolated anti-HBc was significantly higher than the age of patients with both a positive anti-HBc and anti-HBs. Previous studies suggest that with increasing age, the decline in anti-HBs titers is the most likely explanation for an isolated anti-HBc result.23,24 This supports the conception that every patient with an isolated anti-HBc positive result should be considered as at risk for HBVr since it is difficult to be sure that it reflects a false-positive test result.
The key question of this study was to evaluate the result of implementation of the routine HBV screening protocol. Before introduction of the protocol, one HBVr occurred in our cohort and one patient was started on prophylactic antiviral therapy; after protocol implementation, no HBVr occurred and one patient was started on prophylactic antiviral therapy. Before introduction of the protocol, an anti-HBc prevalence of 21.6% was identified, while after implementation of the protocol the anti-HBc prevalence was 14.1%. This is explained by a selection effect: before the HBV screening protocol, a selection of patients was screened who were considered at-risk for HBV. However, without the HBV screening protocol, patients will be missed who are not directly identified as at-risk for HBV. In this study cohort, without the HBV screening protocol we would have missed four patients who tested anti-HBc positive at screening, because they were born in a non-endemic country and were not receiving high-risk medication, and therefore not considered as at-risk for HBV(r). Since the introduction of the protocol we had a standard follow-up protocol with liver serum transaminases and HBV-DNA to detect early signs of HBVr.
A study investigating the cost-effectiveness of routine HBV screening in a hypothetical model of patients who start chemotherapy for solid tumors reports that it is not likely to be cost-effective.25 But the most recent guidelines of the Centers for Disease Control and Prevention, The American Association for the Study of the Liver and the European Association for the Study of the Liver all recommend universal screening despite not being cost-effective.2,9,12,13 Given the low costs of HBV screening, the high additional costs and clinical consequences for patients with HBVr, we recommend routine HBV screening for all patients receiving chemotherapy.17
In conclusion, the implementation of routine HBV screening for all patients treated with chemotherapy increases the number of patients identified as at-risk for HBVr and contributes to prevention of HBVr. The high prevalence of anti-HBc-positive patients in this study indicates the importance of routine HBV screening. To achieve a 100% screening rate, patients must only be allowed to start chemotherapy when the HBV test results are known, for example with an automated warning system to check HBV status before chemotherapy prescription. A cultural change for routine HBV screening and knowledge about the existing guidelines will be a first step to increase the screening rate in patients treated with chemotherapy.
ACKNOWLEDGEMENTS
We wish to thank Mieke Vink-de Goeij and Frank Kristel for providing the database with all patients that received chemotherapy at the Medical Centre Slotervaart.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES