AbstractFull textPDF
Full text
KEY WORDS
Postural orthostatic tachycardia syndrome, orthostatic intolerance
INTRODUCTION
Postural orthostatic tachycardia syndrome (POTS) is a condition in which a change from a supine to an upright position causes an abnormally large increase in heart rate.1 A POTS diagnosis is made when patients meet all criteria shown in table 1. Common complaints, not all explained by the increase in heart rate only, are lightheadedness, palpitations, (pre-)syncope, fatigue, tremulousness and weakness or heaviness (especially of the legs).2,3 POTS is probably underdiagnosed due to the heterogeneity in both presentation and etiology, and therefore the prevalence of POTS is still unsure. Three studies report a prevalence of approximately 170/100,000 in the United States.4-6 Mean age of onset of POTS is approximately 30 years and most patients are between the ages of 20-40 years. There is a clear overrepresentation of women with a corresponding female:male ratio of 5:1.6 We describe two illustrative cases, followed by considerations regarding pathophysiology and treatment consisting of lifestyle advice. This advice may include psychological interventions which, if necessary, may be combined with pharmacotherapy.
CASE REPORTS
Case 1
A 24-year-old Caucasian woman with an unremarkable medical history was referred to the outpatient clinic of internal medicine. Her referring cardiologist suspected POTS based on complaints of vertigo and posturaldependent sinus tachycardia; the patient had no severe orthostatic hypotension and a structurally normal heart as imaged by echocardiography. Her complaints, which also included flushing, malaise and concentration problems, started approximately three months earlier and improved when lying down and worsened when standing. Her sister and father had similar symptoms at the same age. We performed a tilt table test. Her supine blood pressure was 141/84 mmHg and her heart rate was 111 beats per minute (bpm). After being tilted, physical complaints such as flushing and palpitations developed progressively and after seven minutes, the test was stopped. Her heart rate rose to 150 bpm while blood pressure increased slightly to 148/96 mmHg. At this point, blood tests were conducted. Serum noradrenaline level was 887 pg/ml (baseline 292 pg/ml) and adrenaline level was 509 pg/ml (baseline 44 pg/ml). These complaints and findings, in absence of another explanation such as adrenal insufficiency, confirmed the diagnosis of POTS (table 1). Since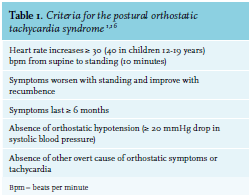 she had such a severe rise in heart rate and two first-degree relatives had similar symptoms, whole exome sequencing was performed after pretest counseling. No pathogenic variant, not even in the SLC6A2 gene (a gene that causes orthostatic intolerance),7 was identified. We advised lifestyle changes, including substantial fluid and salt intake, compression stockings and supervised physical reconditioning (horizontally; for example, cycling). Simultaneously, as part of the recommended dual policy of physical and psychological treatment, we referred her to a psychologist to discuss psychological factors that could contribute to her POTS symptoms.3,8-10 As her symptoms did not improve within weeks, we prescribed short-acting beta blocker propranolol (uptitrated to 40 mg three times a day) and fludrocortisone (62.5 mcg once daily), combined with extra salt (NaCl tablets, 1000 mg three times a day). This resulted in good response, but the fludrocortisone led to severe insomnia. The patient herself suggested modafinil (100 mg bidaily) after reading Raj et al.,1 which was prescribed for her difficulty with concentration. This improved her so-called ‘’brain fog’’.1 For exceptional occasions such as her wedding, she was prescribed desmopressin (DDAVP) after a test-dose including control of sodium levels was conducted, which improved her symptoms substantially. Over time, and considering all lifestyle measures, her condition improved and medication could be tapered after six months to propranolol 40 mg three times a day and salt suppletion only. Currently, her symptoms are well under control with lifestyle measures and propranolol has been further tapered.
she had such a severe rise in heart rate and two first-degree relatives had similar symptoms, whole exome sequencing was performed after pretest counseling. No pathogenic variant, not even in the SLC6A2 gene (a gene that causes orthostatic intolerance),7 was identified. We advised lifestyle changes, including substantial fluid and salt intake, compression stockings and supervised physical reconditioning (horizontally; for example, cycling). Simultaneously, as part of the recommended dual policy of physical and psychological treatment, we referred her to a psychologist to discuss psychological factors that could contribute to her POTS symptoms.3,8-10 As her symptoms did not improve within weeks, we prescribed short-acting beta blocker propranolol (uptitrated to 40 mg three times a day) and fludrocortisone (62.5 mcg once daily), combined with extra salt (NaCl tablets, 1000 mg three times a day). This resulted in good response, but the fludrocortisone led to severe insomnia. The patient herself suggested modafinil (100 mg bidaily) after reading Raj et al.,1 which was prescribed for her difficulty with concentration. This improved her so-called ‘’brain fog’’.1 For exceptional occasions such as her wedding, she was prescribed desmopressin (DDAVP) after a test-dose including control of sodium levels was conducted, which improved her symptoms substantially. Over time, and considering all lifestyle measures, her condition improved and medication could be tapered after six months to propranolol 40 mg three times a day and salt suppletion only. Currently, her symptoms are well under control with lifestyle measures and propranolol has been further tapered.
Case 2
A 44-year-old Caucasian male visited the general practitioner with complaints of syncope while standing. These complaints were present for approximately five to six months and started after an intentional weight reduction of 30 kg (weight at presentation: 95 kg, height:1.97 m). Simultaneously, he developed paresthesia of his legs. He was referred to an internist and a cardiologist for further investigation. The cardiologist excluded underlying cardiac pathology. The internist referred him to our hospital for further diagnostics, in particular, a tilt table test. This was performed, and showed an increase in heart rate from 58 bpm in supine position to 90 bpm when tilted, whereas his blood pressure remained around 154/98 mmHg. Noradrenaline rose to 229 pg/ml from a relatively low baseline level of 69 pg/ml. These measurements fit the criteria of POTS (table 1). The neurologist we consulted in our center concluded the paresthesia to be meralgia paresthetica of the right femoral cutaneous nerve and the left peroneal nerve, possibly triggered by the patient’s weight loss. An association with POTS was excluded, although no biopsy was performed to rule out small fiber neuropathy.11 We advised lifestyle changes, including intake of sufficient fluids and salts and prescribed sodium (NaCl tablets, 1000 mg three times a day). The symptoms of the patient resolved and no further pharmacotherapy was required.
DISCUSSION
This paper describes two illustrative cases of patients with POTS. POTS, first described by Jacob Mendes Da Costa in 1871, is a clinical syndrome and not a distinct disease entity, and has clinical overlap with chronic fatigue syndrome and Ehlers-Danlos syndrome.12 Clinical diagnostic criteria for POTS are provided in table 1.
Pathophysiology
Under normal circumstances, heart rate and blood pressure remain stable or change only slightly and for a very short period of time in response to changing from a supine to an upright position due to a rapid response originating from the baroreceptors. In POTS patients however, heart rate increases to very high levels and for a longer time period. This is presumably due to different pathways. Hypovolemia is present in two-third of patients with POTS, potentially due to less responsiveness of the renin–angiotensin–aldosterone system.13,14 Elevated (> 600 pg/ml) catecholamine levels upon standing are commonly recognized in patients with POTS.15 Poor exercise tolerance and deconditioning is also present in the majority of cases. Although this could be a cause or a consequence of POTS, the fact that most patients benefit from exercise is an extra argument that deconditioning is a causal factor.16 In addition to these common findings, two specific subtypes can be distinguished in most studies: the hyperadrenergic and the neuropathic subtypes, although in clinical practice this subdivision is less useful and difficult to differentiate.1,9,16 A vast majority of POTS patients experience autonomic dysfunction in various autonomic domains.17 Additional testing, such as measurement of catecholamines in response to standing or assessment of small fiber neuropathy, should be preserved for research purposes only or in specific indications – such as in case 2, where a relationship with the tremendous weight loss was likely.
Hyperadrenergic phenotype
A hyperadrenergic state, present in approximately 50% of patients with POTS, is due to excessive sympathetic discharge characterized by a supraphysiological rise in plasma levels of noradrenaline to 600 pg/ml or higher in response to standing as seen in case 1.3,9,16 Blood pressure may fluctuate or increase heavily (“orthostatic hypertension”) during prolonged standing. Symptoms of stress, emotional behavior and cold pale skin may occur upon standing.1 Likewise, the episodes can also be triggered by emotional stimuli and physical activity.3 Earlier described hypovolemia may also attribute to the hyperadrenergic state. Hyperthyroidism or catecholaminesecreting tumors should be ruled out as alternative diagnoses in patients presenting with this phenotype. In rare cases of familiar occurrence of POTS, a heterozygous variant in the SLC6A2 gene encoding the norepinephrine transporter has been found.7 Hyperadrenergic states have also been suggested to be secondary to immune disorders associated with antibodies against components of the voltage-gated potassium channel complex.3 Autoantibodies against the nicotinic acetylcholine receptor have been described to correlate with the severity of autonomic dysfunction in small patient cohorts.15,18,19 Recent studies have shown elevated autoantibodies against adrenergic receptors (α1AR) in patients with POTS, resulting in a compensatory autonomic vasoconstriction and concurrent α1AR-mediated tachycardia.20,21 Furthermore, another study showed Angiotensin II Type 1 Receptor autoantibodies (AT1R) in POTS patients.22 However, these are small studies in selected patient populations and therefore further research is needed to establish the clinical implications.
Neuropathic phenotype
The other important mechanism found in POTS is presumably caused by (partial) peripheral sympathetic denervation leading to impaired peripheral vasoconstriction.23 This denervation is thought to be a consequence of a small fiber neuropathy, which may be diagnosed by biopsy, impaired sweat testing or sudomotor axon reflex testing.11,15 There is lack of vasoconstriction resulting in venous pooling in the lower limbs, which is reversed when the patient lies down as a result of gravity.2,3 Considering these aspects, the second case is expected to have the neuropathic form of POTS potentially related to his weight loss24,25 although biopsy was not performed. Indeed, in a small study over one third of patients fulfilled the criteria for POTS after bariatric surgery.26
Diagnostic approach
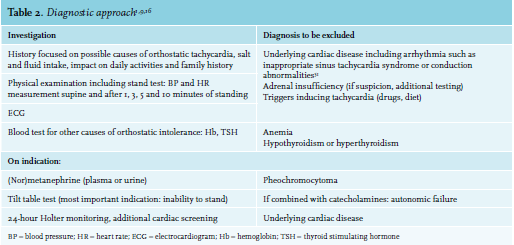
POTS patients present with atypical and rather common symptoms. The diagnostic approach is therefore challenging and based on four criteria (table 1) while excluding other causes (table 2). A key symptom for establishing the diagnosis of POTS within the differential diagnosis is worsening of symptoms while standing up. Since epidemiology and symptoms may overlap, inappropriate sinus tachycardia and vasovagal syncope must be distinguished from POTS, although these diagnoses are not mutually exclusive.16 Any condition or drug that could be causing orthostatic tachycardia, such as dehydration or pheochromocytoma, should be identified and adequately treated.27 The tilt table test is commonly used for diagnosing POTS, although this is not strictly necessary: a simple stand test might be sufficient to confirm the diagnosis; the same is true for the measurement of catecholamines before and after tilting.1,9,16
Overlap with other conditions
There seems to be an overlap with fibromyalgia (FM) and other medically unexplained physical symptoms (e.g., chronic fatigue sleep disturbances).3,28-30 POTS is found in up to 50% and 60% of patients with chronic fatigue syndrome (CFS) and FM, respectively.5,31 In patients with CFS, abnormalities of the vascular and autonomic nervous system are common.5,31 Similar to POTS patients, small fiber neuropathy also affects a majority of FM patients.32,33 Given the similarities between symptoms of FM, CFS and POTS, it is reasonable to assume shared etiology between these conditions.28,31 This may involve so-called “somatic hypervigilance” or “central sensitization,” in which relatively mild or routine sensory information is experienced more intensely or more distressing than usual.10,34-37 This may also lead to a stronger physiological response to exercise, often reason to quit exercising.38
Treatment options
Currently, there is no standard treatment for POTS, and treatment strategies should be based on clinical presentation, the assumed underlying pathophysiology, potential overlapping syndromes, deconditioning and any psychological factors that can sustain symptoms. First-line POTS therapy consists of lifestyle recommendations. A multidisciplinary approach including physiotherapy and psychological support is recommended to optimize lifestyle treatment to avoid overmedicalization (table 3).3,8,9,16
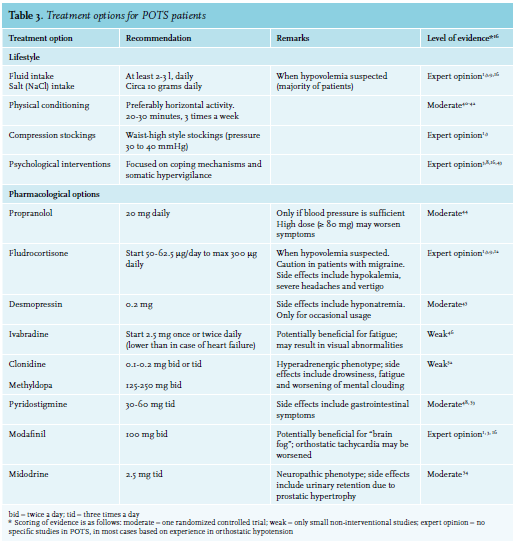 Since hypovolemia seems to play a major role in the majority of patients, fluid intake of at least 2-3 liters as well as 10 grams of salt per day (studies differ in their advice between 8-10 or 10-12 grams) should be advised to prevent hypovolemia.1,16,27,39 A 24-hour urine measurement of sodium can be helpful since most patients often overestimate their current salt intake. Most patients may benefit from wearing support garments such as thigh-or waist-high tight support stockings in accordance with recommendations for orthostatic hypotension.1,3 Patients should be encouraged to begin a gradual program of physical reconditioning under supervision of a dedicated physical therapist, working toward a goal of performing 20 to 30 minutes of aerobic activity (preferably horizontal, e.g., cycling) three times a week.40-42 Psychological treatment including psychotherapy can be helpful, both to improve coping mechanisms as well as to address the somatic hypervigilance.8,43 In our center, every POTS patient is offered a visit to a psychologist. The psychologist can assess to which extent psychological issues may be involved in the etiology or maintenance of POTS.8 Clinical trials to identify the most effective psychological treatment enabling more specific referral and treatment are needed. Pharmacotherapy may be required for patients who remain symptomatic after three months of optimal lifestyle interventions, or for patients whose severe symptoms hamper life style modifications even at earlier stages. Several drugs have shown a positive effect in POTS treatment, although one should keep in mind that the highest level of evidence is moderate and most options are based on non-interventional studies or expert opinions only (table 3). The most relevant options are shortly described below. The best available evidence exists for low doses of short-acting beta blockers, in particular propranolol. It is mainly effective at lowering standing heart rate and improving complaints of palpitations.3,8,9,16 Interestingly, in a direct comparison, propranolol was inferior to exercise therapy.42 In this study, the combination of exercise and propranolol was not studied, contrasting with our recommendation to first optimize lifestyle before considering pharmacotherapy. High doses (≥ 80mg) of propranolol fail to show further improvement and may even worsen symptoms.44 Fludrocortisone, a mineralocorticoid, can be used when hypovolemia is suspected, to enhance sodium retention and to promote intravascular volume expansion.1 However, it can exacerbate headaches and vertigo, particularly in patients with migraine.3 Incidentally, desmopressin can be used to reach rapid volume expansion.45 Ivabradine, a selective sinus node inhibitor can slow heart rate without effecting blood pressure and seems to have a beneficial effect on fatigue.46,47 When symptoms are severe due to high sympathetic nervous system activity, central sympatholytic agents, clonidine and methyldopa, can be perscribed. In patients who are refractory to other commonly-used medications, the use pyridostigmine, a peripheral acetylcholinesterase inhibitor, can be considered.48 Stimulating agents such as modafinil or methylphenidate may be considered to improve concentration and reduce mental clouding, although its mechanism is unknown. One should keep in mind that modafinil may aggravate the orthostatic tachycardia since tachycardia is a well known side effect, although this was not shown in a small trial focused on safety in patients with POTS.1,49 The peripheral α1-adrenergic agonist midodrine may elicit vasoconstriction by reducing venous pooling, especially in neuropathic POTS.23 As most POTS patients are between 20 and 40 years of age, its major side effect (e.g., urinary retention due to prostatic hypertrophy) is not an issue.2,6
Since hypovolemia seems to play a major role in the majority of patients, fluid intake of at least 2-3 liters as well as 10 grams of salt per day (studies differ in their advice between 8-10 or 10-12 grams) should be advised to prevent hypovolemia.1,16,27,39 A 24-hour urine measurement of sodium can be helpful since most patients often overestimate their current salt intake. Most patients may benefit from wearing support garments such as thigh-or waist-high tight support stockings in accordance with recommendations for orthostatic hypotension.1,3 Patients should be encouraged to begin a gradual program of physical reconditioning under supervision of a dedicated physical therapist, working toward a goal of performing 20 to 30 minutes of aerobic activity (preferably horizontal, e.g., cycling) three times a week.40-42 Psychological treatment including psychotherapy can be helpful, both to improve coping mechanisms as well as to address the somatic hypervigilance.8,43 In our center, every POTS patient is offered a visit to a psychologist. The psychologist can assess to which extent psychological issues may be involved in the etiology or maintenance of POTS.8 Clinical trials to identify the most effective psychological treatment enabling more specific referral and treatment are needed. Pharmacotherapy may be required for patients who remain symptomatic after three months of optimal lifestyle interventions, or for patients whose severe symptoms hamper life style modifications even at earlier stages. Several drugs have shown a positive effect in POTS treatment, although one should keep in mind that the highest level of evidence is moderate and most options are based on non-interventional studies or expert opinions only (table 3). The most relevant options are shortly described below. The best available evidence exists for low doses of short-acting beta blockers, in particular propranolol. It is mainly effective at lowering standing heart rate and improving complaints of palpitations.3,8,9,16 Interestingly, in a direct comparison, propranolol was inferior to exercise therapy.42 In this study, the combination of exercise and propranolol was not studied, contrasting with our recommendation to first optimize lifestyle before considering pharmacotherapy. High doses (≥ 80mg) of propranolol fail to show further improvement and may even worsen symptoms.44 Fludrocortisone, a mineralocorticoid, can be used when hypovolemia is suspected, to enhance sodium retention and to promote intravascular volume expansion.1 However, it can exacerbate headaches and vertigo, particularly in patients with migraine.3 Incidentally, desmopressin can be used to reach rapid volume expansion.45 Ivabradine, a selective sinus node inhibitor can slow heart rate without effecting blood pressure and seems to have a beneficial effect on fatigue.46,47 When symptoms are severe due to high sympathetic nervous system activity, central sympatholytic agents, clonidine and methyldopa, can be perscribed. In patients who are refractory to other commonly-used medications, the use pyridostigmine, a peripheral acetylcholinesterase inhibitor, can be considered.48 Stimulating agents such as modafinil or methylphenidate may be considered to improve concentration and reduce mental clouding, although its mechanism is unknown. One should keep in mind that modafinil may aggravate the orthostatic tachycardia since tachycardia is a well known side effect, although this was not shown in a small trial focused on safety in patients with POTS.1,49 The peripheral α1-adrenergic agonist midodrine may elicit vasoconstriction by reducing venous pooling, especially in neuropathic POTS.23 As most POTS patients are between 20 and 40 years of age, its major side effect (e.g., urinary retention due to prostatic hypertrophy) is not an issue.2,6
Quality of life
POTS patients are limited in their physical activities and can become deconditioned over time.3,12,42 Unsurprisingly, quality of life in patients with POTS is low. Benrud-Larssen et al. reported that patients with POTS and patients with congestive heart failure had comparable physical and psychological composite scores.34 No correlation was found between quality of life and the maximal increase in heart rate.43 Despite the low quality of life, the prognosis of POTS is favorable, since 60% of the patients return with the given lifestyle and pharmacological options within five years to their level of functioning before onset; this should be emphasized to patients.2,50 However, resolution of symptoms as illustrated in the patients above is not always the case, and may lead to a more complex and chronic condition frustrating both patient and physician.
CONCLUSION
In conclusion, POTS is a heterogeneous clinical syndrome that overlaps with multiple syndromes such as chronic fatigue syndrome and Ehlers-Danlos syndrome. The diagnosis can be made in most cases by a thorough history, physical examination and a limited amount of additional testing to rule out other causes of orthostatic intolerance. Currently, there is not one standard treatment, but a treatment plan should entail lifestyle recommendations and psychological treatment. Pharmacological treatment is reserved for the patients who remain symptomatic despite these interventions. Especially in current times of self-diagnosing and ‘self-educated’ patients who are familiar with this syndrome, clinicians should not only be well informed and aware of POTS, but also familiar with its multifactorial background and treatment options in order to optimize therapy options for their patients.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received. Both patients provided written informed consent for publication.
REFERENCES
- Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127:2336-42.
- Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R. Postural tachycardia syndrome--current experience and concepts. Nat Rev Neurol. 2011;8:22-34.
- Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214-25.
- Goldstein DS, Robertson D, Esler M, Straus SE, Eisenhofer G. Dysautonomias: clinical disorders of the autonomic nervous system. Ann Intern Med. 2002;137:753-63.
- Schondorf R, Benoit J, Wein T, Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75:192-201.
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. 2009;20:352-8.
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541-9.
- Raj V, Opie M, Arnold AC. Cognitive and psychological issues in postural tachycardia syndrome. Auton Neurosci. 2018.
- Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome – Diagnosis, physiology, and prognosis. Auton Neurosci. 2018.
- Khurana RK. Visceral sensitization in postural tachycardia syndrome. Clinical autonomic research: official journal of the Clinical Autonomic Research Society. 2014;24:71-6.
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One. 2013;8:e84716.
- Garland EM, Celedonio JE, Raj SR. Postural Tachycardia Syndrome: Beyond Orthostatic Intolerance. Curr Neurol Neurosci Rep. 2015;15:60.
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574-82.
- Mustafa HI, Garland EM, Biaggioni I, et al. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 2011;8:422-8.
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308-13.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41-63.
- Rea NA, Campbell CL, Cortez MM. Quantitative assessment of autonomic symptom burden in Postural tachycardia syndrome (POTS). J Neurol Sci. 2017;377:35-41.
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847-55.
- Watari M, Nakane S, Mukaino A, et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol. 2018;5:486-92.
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:e000755.
- Fedorowski A, Li H, Yu X, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. 2017;19:1211-9.
- Yu X, Li H, Murphy TA, et al. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. J Am Heart Assoc. 2018;7(8).
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008-14.
- Sherman DG, Easton JD. Dieting and peroneal nerve palsy. JAMA. 1977;238:230-1.
- Landais A. Neurological complications of bariatric surgery. Obes Surg. 2014;24:1800-7.
- Ponnusamy V, Owens AP, Purkayastha S, Iodice V, Mathias CJ. Orthostatic intolerance and autonomic dysfunction following bariatric surgery: A retrospective study and review of the literature. Auton Neurosci. 2016;198:1-7.
- Grubb BP. Postural tachycardia syndrome. Circulation. 2008;117:2814-7.
- Staud R. Autonomic dysfunction in fibromyalgia syndrome: postural orthostatic tachycardia. Curr Rheumatol Rep. 2008;10:463-6.
- Lewis I, Pairman J, Spickett G, Newton JL. Clinical characteristics of a novel subgroup of chronic fatigue syndrome patients with postural orthostatic tachycardia syndrome. J Intern Med. 2013;273:501-10.
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7:204-10.
- Sharif K, Watad A, Bragazzi NL, et al. On chronic fatigue syndrome and nosological categories. Clin Rheumatol. 2018;37:1161-70.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain: a journal of neurology. 2013;136(Pt 6):1857-67.
- Levine TD, Saperstein DS. Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia patients. Clin Rheumatol. 2015;34:413-7.
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531-7.
- Benrud-Larson LM, Sandroni P, Haythornthwaite JA, Rummans TA, Low PA. Correlates of functional disability in patients with postural tachycardia syndrome: preliminary cross-sectional findings. Health Psychol. 2003;22:643-8.
- Masuki S, Eisenach JH, Johnson CP, et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol. (1985) 2007;102:896-903.
- Fleming KC, Volcheck MM. Central sensitization syndrome and the initial evaluation of a patient with fibromyalgia: a review. Rambam Maimonides Med J. 2015;6:e0020.
- Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18:300-7.
- Z’Graggen WJ, Hess CW, Humm AM. Acute fluid ingestion in the treatment of orthostatic intolerance – important implications for daily practice. Eur J Neurol. 2010;17:1370-6.
- Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. 2012;590:3495-505.
- Winker R, Barth A, Bidmon D, et al. Endurance exercise training in orthostatic intolerance: a randomized, controlled trial. Hypertension. 2005;45:391-8.
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58:167-75.
- Moon J, Kim DY, Byun JI, et al. Orthostatic intolerance symptoms are associated with depression and diminished quality of life in patients with postural tachycardia syndrome. Health Qual Life Outcomes. 2016;14:144.
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725-34.
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart rhythm. 2012;9:1484-90.
- Gee ME, Watkins AK, Brown JN, Young EJA. Ivabradine for the Treatment of Postural Orthostatic Tachycardia Syndrome: A Systematic Review. Am J Cardiovasc Drugs. 2018;18:195-204.
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011;13:427-30.
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience. Pacing Clin Electrophysiol. 2011;34:750-5.
- Kpaeyeh J, Jr., Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of postural tachycardia syndrome: a randomized, placebo-controlled trial. J Clin Psychopharmacol. 2014;34:738-41.
- Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74:1106-10.
- Agarwal AK, Garg R, Ritch A, Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-80.
- Gaffney FA, Lane LB, Pettinger W, Blomqvist CG. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest. 1983;83(2 Suppl):436-8.
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734-40.
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebocontrolled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond). 2014;126:289-96



