

KEYWORDS
Donation after circulatory death, organ donation, organ transplantation
INTRODUCTION
Organ donation after circulatory death (DCD), in addition to donation after brain death (DBD), is one of the ways to tackle the growing demand for organs for transplantation. The Netherlands was one of the first European countries to transplant organs (kidneys) from DCD donors, starting in the early 1980s.1,2 DCD in the Netherlands is supported by legislation on organ donation and a national DCD protocol was introduced to standardise the DCD procedures.
The majority of DCD donors in the Netherlands are controlled DCD. According to the Maastricht criteria (table 1) these donors are of category 3 (cDCD3), patients with an infaust medical prognosis, where treatment will be withdrawn awaiting circulatory arrest.3 Since February 2001, kidneys from both DBD and DCD donors have been indiscriminately allocated through the standard national renal allocation program. Although there is a higher risk for primary non-function or delayed graft function after cDCD kidney transplantation, the small difference in graft survival after cDCD compared with DBD kidney transplantation turned out to be acceptable.2,4,5
In practice, patients in intensive care units (ICU) with a non-recoverable or irreversible neurological injury, not meeting brain death criteria, in whom the medical decision to withdraw treatment is taken, are candidates for cDCD and can be referred to the organ procurement organisation. According to the Dutch protocol, cDCD is possible when there is an expected time between withdrawal of life-supporting treatment and circulatory arrest (agonal phase) of two hours or less for kidneys and one hour or less for liver, lungs and pancreas. During this time the organ procurement team is stand-by. After circulatory arrest a no-touch period of five minutes is maintained after which the donor is transported to the operating theatre to procure the organs.
Although the cDCD program predominantly concerned kidney transplantation, the Netherlands has also been successfully transplanting livers from DCD donors since 1999, and DCD lungs and pancreas since 2005.2,6,7 The question raised by the increasing number of DCD donors is how it affects the donation program in terms of effort and number of renal as well as non-renal transplantations. In this article we describe 15 years of experience, between 2000 and 2014, of cDCD in the Netherlands, starting with the number of organ donors who entered into the deceased donation program (referred donors). We focussed on the number of donors from which no organ was recovered or transplanted after referral and which limitations were encountered in cDCD donation. Finally we show the effect on the number of transplantations.
METHODS
We extracted data regarding deceased organ donation and transplantation in the Netherlands, during the years 2000-2014, from the organ donor procurement registration of the Dutch Transplant Foundation. First we evaluated the total number of referred DBD, cDCD (Maastricht category 3 and 4) and uncontrolled DCD (Maastricht category 1 and 2) donors per year. Referred donors are defined as potential donors in whom at least one organ is reported to the organ procurement organisation with the intention to donate.
From the referred DBD and cDCD3 (Maastricht category 3 only) donors we calculated the percentage of donors whose organs were recovered for transplantation (actual donors) and the percentage of donors from whom at least one organ was used for transplantation (utilised donors, see figure 1). These calculations were done per three-year periods, in order to avoid fluctuations that are present in analyses per year. Reported reasons for no procurement or no transplantation of organs were evaluated. We also analysed these numbers for different age groups. The numbers of transplanted DBD and DCD organs from Dutch donors were expressed per organ type, in which paired organs (kidneys and lungs) were counted as two. The mean number of organs transplanted per referred donor for cDCD and DBD donors were compared per year, with paired organs and split livers being counted as two. Differences in percentages of utilised donors between DBD and DCD, time periods, and age groups were statistically tested by chi-square test using IBM SPSS23 software.
To collect more information about the reasons for not procuring organs in cDCD donors after referral, we used data from another application, our national medical record review. In this application donation officers and transplant coordinators enter data from all patients who died in the ICU regarding organ donation, from identification of potential organ donors until organ procurement.8 The database is almost complete for the years 2008-2014, covering 90% of all 1045 referred cDCD donors in the Netherlands in 2008-2014. From 941 referred potential cDCD donors in this period who gave consent for donation, we evaluated the reasons for not procuring the organs.
RESULTS
Donors
The total number of deceased patients in the Netherlands who were referred for organ donation increased by 58%, from 213 donors in 2000 (13.4 per million population) to 336 donors (20.0 per million population) in 2014 (figure 2). While DBD donor referral fluctuated between 111 and 170 donors (mean 137 donors) per year, cDCD donor referral has grown from 36 to 181 donors yearly in these 15 years. This represented a change in the percentage of cDCD among all donor referrals of 14% in 2000 to 54% in 2014.
The total numbers of actual and utilised donors did not keep up with the numbers of donors referred, and increased less, at 34% for both. Over the past 15 years, the percentage of referred organ donors that became actual or utilised donors was significantly lower in cDCD3 than in DBD (p < 0.001). In DBD this utilisation rate fluctuated per three-year period between 95% and 97%, but it decreased in cDCD3 from 84% in the years 2000/2002 to 67% in 2012/2014 (p < 0.001, figure 3). Because the contribution of cDCD3 donors among deceased donors rose, the total percentage of utilised donors dropped in the Netherlands, from 95% in 2000 to 81% in 2014.
The number of cDCD3 donors predominantly increased in the higher age groups in recent years, especially since the donor age limit for DCD was raised from 65 to 75 years nationwide in the year 2011 (figure 4A). However, organs were less often recovered and transplanted from the older age groups of referred cDCD3 donors (figure 4B). The percentage of utilised donors was 86% in donors aged 0-40 years in contrast to 62% in donors aged 66-75 years (p < 0.001).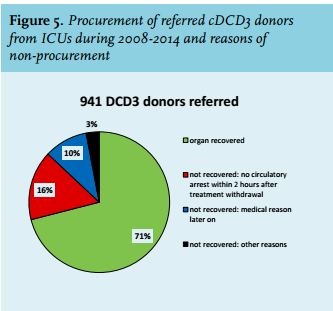
Medical reasons were predominantly reported as the reason for not procuring organs from referred donors. After procurement, organs were not transplanted because of newly discovered medical reasons or anatomical pathology of the organ.
Our medical chart review database of potential donors who died in the ICU between 2008 and 2014 also showed another reason for non-procurement. In 71% of the 941 referred potential cDCD donors with consent for donation the organs were recovered. In 16%, procurement was not possible because donors did not die within the maximum two-hour time limit interval after withdrawal of life-supporting treatment (figure 5). In 10% of the referred cDCD donors, organs were not recovered because of other medical reasons that appeared after referral (for example abnormal features in organs discovered by imaging or lab).
Transplantations
Thus far the introduction of cDCD3 in the Netherlands has been successful. The number of referred donors rose considerably (58%, figure 1) and the total number of organs that are finally transplanted increased by 42%, from 646 in 2000 to 920 in 2014. The number of transplanted kidneys was higher during the years 2012-2014, compared with the three-year periods before 2012, and the numbers of transplanted livers and lungs steadily grew, all due to cDCD3 (figure 6). However, so far this has not resulted in higher numbers of transplanted organs per referred DCD donor. These numbers fluctuated yearly with between 1.6 and 2.0 organs per donor, numbers that are still lower than in DBD donors (varying between 3.3 and 4.1 organs per referred donor). cDCD still results predominantly in kidney donation and less often in non-renal donation (liver, lung, pancreas). And until now no DCD heart donation has been performed in the Netherlands.
DISCUSSION
The DCD program in the Netherlands was initiated to stimulate the total number of organ transplants from deceased donors by creating an extra pool of donors.1 In the last 15 years, the referral of deceased Dutch organ donors increased significantly (58%), mainly because of cDCD. However, the number of actual and utilised cDCD donors, as well as the total number of organs transplanted, increased less impressively as compared with the number of donors referred. Thus much more effort is needed to stimulate the number of transplants by cDCD donation. Although the number of cDCD livers and lungs is growing, cDCD still results in less non-renal transplants than DBD and as yet heart transplants in the Netherlands.
Developments in the Netherlands regarding cDCD are comparable with those in the United Kingdom (UK) where cDCD has been widely introduced into the donation program. In the UK the number of donors increased by 64% (from 709 to 1164 donors) during the years 2003-2012, which was predominantly the result of the cDCD group growing from 9-43% of all donors.9 Also in the UK there is a discrepancy between the number of referred and transplanted organs from deceased donors. In the UK it was shown that considerably more DCD donors did not result in any organ transplantation after procurement as compared with DBD donors (14% vs. 2% in 2012). Furthermore the proportion of DCD kidneys that were recovered, but not transplanted, grew from 8% to 17% during the years 2003-2012, without any further reasons mentioned.9 A similar development was seen in the Netherlands where an increasing number of referred DCD3 donor organs were not transplanted, even not procured (figure 3B).
In previous studies we reported that an initial higher number of cDCD until 2005 was consistent with a simultaneous lower number of DBD and we postulated the possibility of a ‘substitution’ instead of expansion of the donor pool by DCD.10,11 A similar trend after introduction of DCD was suspected in the UK and to a lesser degree in the United States (US) and in Belgium.2,12,13 It was suggested that potential DBD donors might be recovered as DCD, because of a change in the management of patients with severe brain injury, such as craniostomy, cooling of the patient or possible earlier referral for donation. Summers et al. suggested, however, that the large majority of DCD donors could not originate from potential DBD donors, because the total number of patients who were possibly brain dead had decreased as well.14 This was shown in potential donor audits in the ICU in the UK during 2004-2009. In the US the growing numbers of cDCD donors did not parallel the decrease in DBD donors, but the DCD numbers remained relatively small.15 However, in a hospital region in the US that had relatively more DCD donors, up to 60% of all deceased donors, the number of DBD donors had decreased.12 In Belgium cDCD particularly rose after the year 2005, but it did not increase the total kidney donor rates until 2010.13 The possibility that a further growth in the number of cDCD donors in the Netherlands will be accompanied by a simultaneous decline in the numbers of DBD donors, with as a result less non-renal organs (especially no hearts), is still a matter of concern. cDCD donation is a valuable addition to the donor pool under the condition that it does not substitute the potential DBD pool. We therefore have to stimulate an attitude in hospitals to first wait for brain death determination. The growing number of liver and lung transplants from DCD donors is one of the reasons why the increasing percentage of cDCD donors has not dramatically disturbed the non-renal transplant programs so far, with the exception of heart transplantations, which are still fully dependent on DBD donations in the Netherlands. Also alternative opportunities to create an additional pool of deceased donors should be further explored, such as by stimulating referral of the number of uncontrolled DCD donors (Maastricht category 1 and 2) that cannot interfere with DBD or by stimulating donation from older DBD donors (aged 75 or older).
There are some more limitations to DCD that are responsible for relatively less transplants compared with DBD. One limitation is the warm ischaemia time, which is unavoidable after cessation of treatment in cDCD until death confirmation. The Netherlands has chosen a maximal time limit after withdrawal of life-supporting treatment of two hours for kidney donation and one hour for other organs. This study showed that of the referred cDCD donors, 16% did not die within this two-hour time limit.
In correspondence with our study, Wind et al. showed that 17% of cDCD donors did not die within two hours, and 24% not within one hour after withdrawal of life-supporting treatment. Among these Dutch potential DCD donors median time to death was 20 minutes, but time to death ranged from one minute to 3.8 days.16 Comparable numbers were reported by Saidi et al. and Davila et al., who reported that 30% and 27%, respectively, of intended cDCD donors did not progress to circulatory arrest in one hour after withdrawal of life-supporting treatment.12,17 There are ways to tackle this limitation in cDCD. Reid et al. reported that longer times after withdrawal of life-supporting treatment were associated with greater donor instability, but they also reported that neither patient instability it is important to use universal definitions to evaluate a change in this waiting time properly. On the other hand we could use models to better predict the agonal phase after withdrawal of life-supporting treatment in potential cDCD donors.16,17,20-22 This could prevent starting a laborious intensive and expensive donation procedure, involving transplant coordinators, procurement teams, and preparation of an operation room. It could also prevent a stressful time for a grieving family and disappointment due to an unsuccessful donation procedure.23,24 Although it is hard to find a valid model based on risk factors that will be accurate enough to increase transplantation after donor referral, it is absolutely necessary to continue this research. According to our medical record review data from 2008-2013 another 615 ventilated patients from the ICU had a non-recoverable or irreversible medical status, not meeting brain death criteria, but they were not expected to die within two hours and were even not referred as potential cDCD donors. Some of them could have been donors using an extended waiting time after withdrawal of life-supporting treatment of up to four hours or by using an effective prediction model.
Another limitation in cDCD is that organs from older referred donors were less often transplanted, while especially the referral of older cDCD donors has grown in recent years. This could explain why the proportion of utilised donors in cDCD, which is already smaller as compared with DBD donors, has further decreased in recent years. One might expect that older donors are indeed more often discarded because of poor kidney function or proteinuria. However, we have no information about the exact medical reason for non-procurement.
Also follow-up analysis after transplantation of renal as well as non-renal organs from DCD donors needs to be continued. Analysis of kidney transplantations in the Netherlands has shown significantly decreased survival rates in grafts from DCD3 donors (85.0%) compared with those from DBD donors (93.7%) within the first three months after transplantation.4 However, at 12 months, graft survival still differed by 9% between these groups (83.0% and 92.0%, respectively (p < 0.03)) in favour of DBD.
A solution to stimulate the number of organs suitable for transplantation from DCD donors, especially with respect to older marginal donors, is the improvement of donor management and introduction of new preservation techniques after procurement, such as normothermic regional perfusion and machine perfusion. With respect to kidney and lung donation, developments in this area are promising in the Netherlands.25,26 Recent developments in DCD3 in Australia even show that heart donation is possible by using an ex-vivo Organ Care System machine.27,28 It will be a new challenge to introduce DCD3 heart donation in the Netherlands as well as to shorten the heart waiting list.
In summary this study showed that a nationwide introduction of cDCD requires more efforts in donor activities and combatting limitations to reach more transplantations. More DCD has to be evaluated with care in the coming years, since a further rise in organs that are not transplanted will put pressure on resources, the potential willingness of professionals to invest time and money. Furthermore, it would be hard to explain the fact that organs are declined to the general public.
ACKNOWLEDGEMENTS
We would like to thank all the Dutch donor and transplant centres for their contribution to the data.
DISCLOSURES
The authors declare no conflict of interest. No funding or financial support was received.
REFERENCES