

KEYWORDS
Breast cancer, chemotherapy-induced febrile neutropenia, docetaxel, granulocyte colony-stimulating factor, G-CSF
INTRODUCTION
Febrile neutropaenia (FN) is a common and potentially life-threatening complication of chemotherapy, with a reported overall mortality of up to 10%.1-4 Furthermore, FN is associated with substantial morbidity and costs,4-6 often resulting in treatment delays, dose reductions, and even cessation of treatment;7 all result in poorer outcomes.8-10 Therefore, preventing febrile neutropaenia is of high clinical relevance, especially in the curative setting.
A well-known supportive care intervention is the use of granulocyte colony-stimulating factor (G-CSF), which stimulates the proliferation of neutrophils and thereby minimises the incidence of FN and its associated morbidity and costs.11-14 However, the use of G-CSF itself is relatively expensive (approximately € 1,000 per injection)15 and it is associated with side effects such as thrombocytopaenia and muscle-, joint-, and back pain (1-10%).11,15 Both European and American guidelines recommend the use of primary G-CSF prophylaxis in cases of FN risk ≥ 20%.16-20
As FN rates in randomised clinical trials (RCTs) are significantly lower than in observational studies,21 it is important to study the incidence of FN after specific chemotherapy cycles in daily clinical practice to provide clinicians with clinically applicable recommendations for G-CSF use. However, despite the global and widespread use of chemotherapy, high quality literature on the incidence of FN during specific chemotherapy regimens in daily clinical practice in various cancer types, for example, breast cancer, is scarce.22 In addition, guidelines usually lack advice when to administer G-CSF. Thus, although the 20% cut-off may be clear, it remains unclear when to use primary G-CSF prophylaxis in daily clinical practice.
In our experience, a substantial number of breast cancer patients treated with docetaxel (100 mg/m2) experienced FN during these chemotherapy cycles, which was in concordance with the experience of four regional cancer centres in Ontario, Canada.23 Two systematic reviews also reported median FN rates of 23.9%22 and 30.6%24 in a specific regimen, containing three cycles of FEC (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2 every three weeks followed by three cycles of docetaxel (100 mg/m2) (3F-3D) every three weeks, yet they did not specify in which specific cycles the risk of FN was highest.
In contrast, another widely used regimen consisting of four cycles of AC (doxorubicin 60 mg/m2, cyclophosphamide 600 mg/m2) every three weeks, followed by 12 weekly cycles of paclitaxel (80 mg/m2)(4AC-12P) seems to cause FN in substantially fewer patients, with FN rates during AC cycles ranging from 2.5%25 to 16.1%,26 although literature is scarce.
Recently, Aagaard et al. developed a risk score for febrile neutropenia after chemotherapy (FENCE score), both for the first cycle of chemotherapy27 and for cycles 2-6 in patients with solid cancers.28 This risk score27 is easily available online and requires various patient and chemotherapy characteristics. Although the FENCE score may be a helpful tool, it does not discriminate between different taxane regimens while, in our experience, other taxane regimens such as weekly paclitaxel, almost never cause FN. Thus, simply following the FENCE score may lead to unnecessary administration of G-CSF. Therefore, the question remained whether the use of primary G-CSF prophylaxis would be justified during all or a specific cycle of docetaxel for breast cancer patients.
The primary aim of this study was to provide clinical recommendations for the use of primary G-CSF prophylaxis in breast cancer patients by assessing data from daily clinical practice. We assessed and compared the incidence of chemotherapy-induced FN in breast cancer patients receiving either the 3F-3D or 4AC-12P regimens, both of which are widely used in primary breast cancer care.29,3
MATERIALS AND METHODS
Study population
This study was assessed by the Research Assessment Committee and approved by the board of directors of the Meander Medical Centre.
All breast cancer patients receiving neo-adjuvant or adjuvant chemotherapy with either 3F-3D or 4AC-12P in the Meander Medical Centre between January 1st, 2014 and December 31st, 2015 were identified. Patients receiving alternative chemotherapy regimens were excluded.
For each patient, data on gender, age, tumour characteristics, and type of chemotherapy regimen were obtained from the electronic patient files. Episodes of FN, any subsequent dose delays, and any emergency department visits were identified and analysed, as was the use of G-CSF.
In general, hormone-positive, human epidermal growth factor receptor 2 (HER2)-negative patients were given 3F-3D and triple negative or HER2-positive patients were given 4AC-12P. For HER2-positive patients, the 12 paclitaxel cycles were combined with administration of the monoclonal antibody trastuzumab, which was continued as weekly monotherapy up to a total treatment duration of one year. However, the choice of chemotherapy regimen was determined by the attending medical oncologist, in consultation with the patient; when necessary, in the expert opinion of the medical oncologist, choice of chemotherapy regimen could differ from regional policy. FEC (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2), AC (doxorubicin 60 mg/ m2, cyclophosphamide 600 mg/m2), docetaxel (100 mg/ m2), and paclitaxel (80 mg/m2) were all administered in protocolled doses.
Neutrophil counts were measured one day in advance, or on the day of the planned chemotherapy cycle. Chemotherapy cycles were delayed when neutrophil count was below 1000 cells/mm3. In addition to the protocolled measurements, neutrophil count was only monitored when patients experienced fever during their chemotherapy.
FN was defined as any fever ≥ 38.5°C, reported by the patient or measured in the hospital, in combination with an absolute neutrophil count of < 500 cells/mm3. Patients were instructed to contact and visit the emergency department in any case of fever. When patients visited the emergency department for a possible FN episode, a full physical examination was performed, followed by the collection of blood samples, urine samples, and a chest X-ray to identify a focus of the fever. If patients did indeed experience an FN episode, they were admitted and treated with intravenous antibiotics, according to hospital protocol.
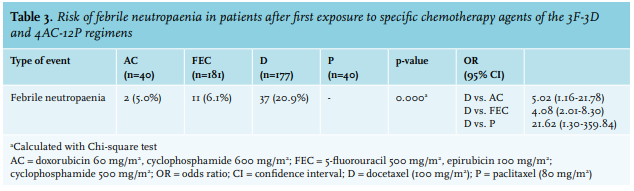
Overall, the incidences of FN in patients undergoing 3F-3D and 4AC-12P were compared. Since trastuzumab is not kwown to cause myelotoxicity, the HER2-positive patients who received 4AC-12P with trastuzumab during the paclitaxel cycles were included in this group in our analyses. Finally, to evaluate the risk of FN during specific chemotherapy cycles, all first administered cycles of AC, FEC, docetaxel, and paclitaxel were compared and odds ratios with a 95% confidence interval were calculated.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics Advanced version 22. Two-sided p-values less than 0.05 were considered statistically significant. Distributions of categorical variables were compared using the Chi-square test, and odds ratios with 95% confidence interval were calculated. Means of continuous variables were compared using the Two-sample t-test.
RESULTS
Patients and tumour characteristics
In total, 227 patients breast cancer patients who received chemotherapy between January 1st, 2014 and December 31st, 2015 were identified. Six patients were excluded for receiving an alternative chemotherapy regimen. These patients received their specific chemotherapy regimen because of comorbidity or previous chemotherapy cycles for prior malignancies. Therefore, a total of 221 patients, all receiving the 3F-3D or 4AC-12P-regimen, were included in the data analyses.
All patients were female with a mean age of 52.9 years (SD ± 9.7 years) (table 1) and no distant metastases were present upon diagnosis, except for one patient who had an oligometastasis in her ileum for which an ileocecal resection was successfully performed.
The 3F-3D-regimen was administered in 181 patients (81.9%). The 4AC-12P-regimen was administered in 40 patients (18.1%); of these 40 patients, 21 received their paclitaxel cycles in combination with trastuzumab. A total of 181 patients were exposed to FEC, whereas 40 patients in total were exposed to AC. All patients within the 4AC-12P group continued with paclitaxel cycles;, resulting in 40 patients who were exposed to paclitaxel. Within the first three cycles of FEC, four patients ceased treatment and thus 177 of 181 patients of the 3F-3D-group were exposed to docetaxel.
Febrile neutropaenia
FN was identified in 61 patients (27.6%) who developed a total of 66 FN episodes. Patients receiving 3F-3D developed significantly more FN episodes during any of their cycles than patients receiving 4AC-12P (31.5% versus 10.0%, OR 4.14, 95% CI: 1.41-12.18) (table 2). Three patients experienced two FN episodes and one patient experienced three episodes, all within the same type of cycles in the 3F-3D group and without G-CSF prophylaxis after their first FN episode. There were no repeated episodes of FN in the 4AC-12P regimen and paclitaxel never caused FN.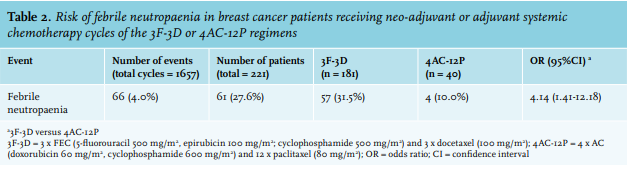
G-CSF was administered in 8.1% of all chemotherapy cycles (135/1657) in 50 patients in total (22.6%). Both pegfilgrastim and lipefilgrastim were used as a G-CSF analogue. The use of G-CSF was not protocolled yet, when prescribed, it was mostly as a secondary prophylaxis with the next cycle of chemotherapy after an FN episode. Four patients (8.0%) received G-CSF as primary prophylaxis due to their age (> 60 years) in combination with a fragile condition. Two episodes of neutropaenia occurred while patients received G-CSF to prevent neutropaenia. However, these two episodes were both not complicated by fever or cycle delay due to neutropaenia and in both cases, neutropaenia was not profound (< 500 cells/mm3).
Focus of infection
The respiratory tract was the most common focus of infection, affecting 13.6% (9/66) of patients; this was a clinical diagnosis without confirmation by a positive culture in all cases. Urinary tract infections and mucositis both occurred separately in 10.6% of FN episodes (both 7/66). Various other foci were identified in 25.8% of FN episodes, for example pneumonia, sinusitis, and ileocolitis. In 39.4% of FN episodes, a focus was not identified (26/66). Overall, pathogens were only isolated in 9.1% of FN episodes (6/66), of which 50.0% (3/6) involved Escherichia coli. FN patients spent a median of five days in the hospital (range: 2-31 days).
Age and central venous catheters
Central venous catheters were identified in a total of 39 patients (17.6%), of whom 20 received 3F-3D and 19 received 4AC-12P. Among patients with a central venous catheter (in both chemotherapy regimens), FN was identified in 28.2%, whereas among patients without a central venous catheter, FN was identified in 27.5% (OR 1.04, 95% CI: 0.480-2.239). The numbers in this study are however, too low to draw any conclusion on whether a central venous catheter increases the risk of FN.
The mean age of patients with and without FN was 52.9 years in both groups (p = 0.988). Of all patients older than 60 years, 27.1% developed FN, whereas 27.8% of patients younger than 61 years developed FN (OR 0.97, 95% CI: 0.50-1.88).
DISCUSSION
As FN rates from RCTs are significantly lower than FN rates from observational studies, it is extremely important to provide FN incidence rates derived from daily clinical practice to provide clinically useful recommendations. These daily clinical practice data are, however, scarce. Therefore, we assessed and compared the incidence of FN in two chemotherapy regimens that are widely used in primary breast cancer care.
We show a high overall incidence (27.6%) for FN in breast cancer patients receiving chemotherapy in our hospital. FN occurred significantly more in patients in the 3F-3D group (31.5%) than in the 4AC-12P group (10.0%) (OR 4.14, 95% CI: 1.41-12.18). This difference seems to be primarily caused by docetaxel (100 mg/m2) within the 3F-3D regimen, as first exposure to docetaxel rendered a significantly higher risk of FN (20.4%) than first exposure to FEC (6.1%), AC (5.0%), or paclitaxel, which never caused FN. This shows that docetaxel poses a high enough risk to justify the use of primary G-CSF, independent of age or World Health Organization (WHO) performance status. In addition, not all taxanes should be considered equally potent in causing FN, considering the absence of FN following paclitaxel administration. This is highly clinically relevant as docetaxel is not only used in breast cancer treatment, but also in other cancer types including in prostate cancer, lung cancer, gastric cancer, and head and neck cancer, although not always as regimen of first choice.15,31
The results of this study raise the question why the 3F-3D treatment regimen, and especially docetaxel within this regimen, would lead to more FN. A possible explanation might be that docetaxel is a more potent cause of neutropaenia. In both treatment regimens, patients received previous cycles of chemotherapy (3 x FEC or 4 x AC), which seemed to be similarly potent causes of FN (6.1% for FEC versus 5.0% for AC) and the first cycles of docetaxel and paclitaxel were only administered when the patient’s bone marrow was sufficiently recovered after the previous chemotherapy cycle, i.e., with a neutrophil count of 1000 cells/mm3 or higher. Another explanation might be that mucositis is a frequently seen side effect of docetaxel cycles. Mucositis causes a potential port d’entrée for bacteria and thus might contribute to a higher FN incidence.
Literature on the incidence of FN in daily clinical practice for different chemotherapy regimens in breast cancer treatment is limited. Bennett et al32 developed a risk stratification of FN for different types of tumours and chemotherapy regimens by using National Comprehensive Cancer Network data. They found that FEC plus sequential docetaxel contributes to an intermediate risk of developing FN (10-20%) in neo-adjuvant or adjuvant systemic chemotherapy treatment of breast cancer patients, which is notably lower than the 31.5% risk of FN that was found in this study.32 As previously described, two large systematic reviews described the incidence of FN without primary prophylactic G-CSF during 3F-3D and found median FN rates of 23.9%22 and 30.6%,24 which resembles the mean FN rate of 31.5% found in this study. Despite these systematic reviews, high-quality evidence remains scarce and to our knowledge, primary G-CSF prophylaxis during 3F-3D is not yet recommended, although national and international guidelines justify this, since the risk of FN is higher than 20%.11-14 This is mostly due to a lack of evidence on the optimal timing of primary G-CSF prophylaxis, either during all cycles of 3F-3D or only during specific cycles. We believe our study addresses this issue by comparing the first exposure to AC, FEC, paclitaxel, and docetaxel and thereby identifying docetaxel as the most potent agent in causing FN.
In addition, literature on FN incidence during 4AC-12P is both insufficient and divergent with FN rates during AC cycles ranging from 2.5%25 to 16.1%.26 Interestingly the 16.1% rate of FN during AC was found by Kim et al,26 who studied the incidence of FN in Korean breast cancer patients receiving four cycles of neoadjuvant and adjuvant AC followed by four cycles of docetaxel. In contrast to our study, they found that 16.1% of patients experienced FN after the first AC cycle and remarkably, only 2.0% of patients experienced FN after the first docetaxel cycle in their treatment regimen.26 It should be noted however, that these data were derived from Asian breast cancer patients and may therefore not be applicable to the Dutch or Western populations of breast cancer patients.
Aagaard et al. recently published a proposed FN risk stratification27,28 which should be welcomed, however, a major limitation is that while this score incorporates certain types of agents (platinums, non-platinum alkylating agents, taxanes, topoisomerase inhibitors, antimetabolites, vinca alkaloids, and other), it does not discriminate between, for example, different types and doses of taxanes. This would result in the same risk score for both a docetaxel and paclitaxel-containing regimen, underscoring our findings that the risk of FN is significantly higher in a docetaxel regimen compared to a paclitaxel regimen, where the risk of FN was zero. Thus, simply following the FENCE score, would result in unnecessary administration of G-CSF, and would subsequently expose patients to unnecessary side effects, in addition to increasing health costs.
This study has several limitations, including its retrospective design, a relatively small sample size, especially for the 4AC-12P group, and single institution focus. However, all hospitals in this region follow the same guidelines and chemotherapy treatment of breast cancer patients is generally analogous in the Netherlands. Moreover, these data reflect the actual situation in daily clinical practice, where the patient population may differ from patient populations in RCTs.21
We are aware that 3F-3D is currently not as widely used as in 2014-2015, while 4AC-12P is increasingly used. Consequently, the unbalanced distribution of both treatment regimens (81.9% 3F-3D vs. 18.1% 4AC-12P) is a major limitation of this study. However, both regimens are still used in clinical practice. We therefore believe that our data remain relevant and address the lack of evidence in optimal timing of primary G-CSF prophylaxis during 3F-3D, which may still benefit patients with breast cancer and possibly other types of cancers.
Finally, we would like to make a remark about the use of primary G-CSF prophylaxis in this study with regard to the observed FN rates. The primary G-CSF prophylaxis in older patients could have masked the incidence of FN, however, this was only the case for four patients. In addition, the incidence of FN could have also been masked by the use of secondary G-CSF prophylaxis during the docetaxel cycles in 11 patients who developed febrile neutropaenia after one of their FEC cycles. Therefore, without both forms of G-CSF prophylaxis, the actual incidence of FN may be even higher.
CONCLUSION
In conclusion, this study with data based on regular clinical practice shows that incorporating docetaxel monotherapy (100 mg/m2) in a neo-adjuvant or adjuvant chemotherapy regimen in the treatment of breast cancer patients renders a high risk of FN compared to a weekly paclitaxel-containing regimen, 31.5% versus 10.0%, (OR 4.14, 95% CI: 1.41-12.18). Our analysis of the docetaxel monotherapy section of the treatment regimen demonstrates that the risk of FN (20.9%) clearly surpasses FN risk following paclitaxel monotherapy (0%), and it is also considerably higher than the FN risk of the anthracycline section in both regimens (~ 5%). It can therefore be concluded that, according to international guidelines, the nearly 21% risk of FN justifies the use of primary G-CSF following docetaxel monotherapy.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
This study was assessed by the Research Assessment Committee and approved by the board of directors of the Meander Medical Centre.
ACKNOWLEDGEMENT
The authors kindly acknowledge Pieternel Pasker-de Jong, epidemiologist in the Meander Medical Centre for her support in the statistical analysis of this study
REFERENCES