

KEYWORDS
Antibiotic resistance, community-acquired pneumonia, empirical antibiotic treatment, healthcare-associated pneumonia
INTRODUCTION
Traditionally, pneumonia is categorized as communityacquired pneumonia (CAP), hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP), as the aetiology and empirical antibiotic treatment differs depending on where and how the infection was acquired. In 2005, healthcare-associated pneumonia (HCAP) was introduced as a novel category by the American Thoracic Society (ATS) and the Infectious Diseases Society of America HAP and VAP guidelines.1 Patients with HCAP often present atinfection with antibiotic-resistant or healthcare-associated pathogens, such as Staphylococcus aureus, Gram-negative Enterobacteriaceae, and Pseudomonas aeruginosa.1 Therefore, the guidelines recommend empirically treating HCAP with broad-spectrum antibiotics, similar to HAP and VAP.1 This has led to a large increase of broad-spectrum antibiotic use without apparent clinical benefit for these patients.2-5 Recent evidence suggests that the predictive value of HCAP criteria for the need of broad-spectrum antibiotic treatment might be lower than anticipated.2,6-11 In response to these findings, HCAP was removed from the 2016 ATS HAP/VAP guidelines and it was suggested to consider incorporating HCAP recommendations into CAP guidelines, as both CAP and HCAP patients are initially cared for in the emergency department.8 Several studies have already evaluated the predictive value of HCAP criteria for bacterial aetiology in CAP patients.6,7 However, the appropriateness of incorporating HCAP into CAP guidelines depends on the prevalence of pathogens requiring broader antibiotic treatment and the preferred empirical treatment for CAP patients, which differs per geographical region. In the Netherlands, the first choice of empirical treatment for moderate-severe CAP is narrow-spectrum beta-lactam monotherapy.12 Current HCAP research focuses on predicting the presence of multidrug-resistant pathogens (including Methicillinresistant Staphylococcus aureus (MRSA) and extendedspectrum beta-lactamase-producing Enterobacteriaceae) and non-susceptibility to broad-spectrum beta-lactams (ceftriaxone or ampicillin-sulbactam), macrolides and fluoroquinolones.13-17 In the Netherlands, infections caused by these resistant pathogens are rare and pneumonia acquired in nursing homes is usually considered as HAP. This is why the relevance and predictive value of HCAP criteria for the Northern European or Dutch setting, i.e. for the non-susceptibility to narrow-spectrum beta-lactams, remains unknown. Our main study objective was to evaluate the predictive value of HCAP criteria for narrow-spectrum beta-lactam (i.e. amoxicillin) non-susceptibility (thus needing broad-spectrum treatment) in patients hospitalized for moderate-severe CAP. In addition, we assessed the predictive value of HCAP criteria for non-susceptibility to broader antibiotic regimens, including amoxicillin-clavulanic acid, ceftriaxone, moxifloxacin, amoxicillin plus azithromycin and amoxicillin plus ciprofloxacin.
MATERIALS AND METHODS
Study subjects and design
We performed a post-hoc analysis of an observational cohort study, nested within the Community-Acquired Pneumonia — Study on the Initial Treatment with Antibiotics of Lower Respiratory Tract Infections (CAP-START trial) — which was a cluster randomised trial performed between February 2011 and August 2013 in seven hospitals in the Netherlands.18 Patients above 18 years of age who were admitted to a non-ICU ward for suspicion of pneumonia were eligible for study participation. The study was approved by the ethics review board of the University Medical Center Utrecht (reference number 10/148). Written informed consent for data collection was obtained within 72 hours after hospital admission.
Data collection
Data on HCAP criteria, co-morbidities, clinical presentation, antibiotic use, complications and clinical outcome were retrieved prospectively from medical records by trained research nurses after patient inclusion. As pneumonia acquired in nursing homes are considered as HAP in the Netherlands, these patients were not included in the original trial. Therefore, the following HCAP definition was used: hospitalization within the last 90 days, residence in long-term care facilities other than nursing homes, receiving wound care or intravenous therapy in the previous 30 days or attending haemodialysis clinics.1
Microbiology
Sputum and blood cultures, urinary antigen tests and antibiotic susceptibility testing were performed as part of routine care. Susceptibility was determined by routinely performed microbiological tests. To account for the possibility of false-positives due to colonization, the causative pathogen per patient was determined, accounting for the specificity of the different microbiological tests, where positive urine antigen tests and blood cultures were assumed to have a higher specificity for causative pathogens than sputum cultures. For example, in a patient with a positive pneumococcal urinary antigen test and S. aureus cultured from sputum, Streptococcus pneumoniae was considered the causative pathogen due to the higher specificity of the urinary antigen test, and S. aureus was considered as colonization. Susceptibility testing was reported as sensitive, intermediate or resistant by participating microbiology laboratories. Intermediate and resistant results were considered as non-susceptible for all the analyses. In patients with multiple possible causative pathogens (i.e. multiple pathogens in sputum culture and no pathogens from blood culture or urinary antigen tests), susceptibility to antibiotics was determined by the most resistant pathogen. In cases of missing susceptibility data, susceptibility per antibiotic was imputed and assumed to be susceptible (S) if the prevalence of resistance to the antibiotic was ≤ 10% in national surveillance data; non-susceptible (R), if the prevalence was ≥ 90%; or unknown (U), if the prevalence was between 10 and 90% (supplementary table S1). Pathogens were considered susceptible (S) to combination antibiotic therapy if susceptible to any of the two antibiotics; unknown (U) if susceptible to one antibiotic and unknown to the other antibiotic or if unknown to both antibiotics; and non-susceptible (R) if non-susceptible to both antibiotics.
Statistical analysis
Descriptive statistics were used to compare baseline characteristics between CAP and HCAP patients. Sensitivity analyses were performed for cases with unknown (U) antibiotic susceptibility which were either assumed to be all susceptible (best-case scenario) or all non-susceptible (worst-case scenario). Predictive values, sensitivity and specificity for non-susceptibility per empirical antibiotic strategy were calculated using 2 x 2 contingency tables. We calculated 95% confidence intervals using the Wilson score interval method.19 Analyses were performed using the Statistical Package for the Social Sciences for Windows (Version SPSS 21.0.0.0). Graphs were created using GraphPad PRISM (Version 7.02).
RESULTS
A total of 2,283 patients with moderate-severe CAP were included in the CAP-START study of which, 527 (23.1%) were classified as HCAP. Among these HCAP patients, 318 (60%) were hospitalized within the last 90 days; 111 (21%) resided in an elderly home; 166 (32%) received intravenous therapy in the previous 30 days; 94 (18%) received wound care in the previous 30 days; and 17 (3%) were on chronic haemodialysis. In comparison to patients with CAP, patients with HCAP were older, had more co-morbidities, had higher disease severity scores (PSI on admission), had higher influenza vaccination rates, were more often dependent on daily living activities (ADL) and more often had treatment restrictions (table 1). Clinical outcomes of patients with HCAP were worse, with higher in-hospital, 30-day and 90-day mortality rates. There were no differences between patients with CAP and HCAP regarding the frequency with which microbiological testing was performed, except for a slightly higher rate of Legionella urinary antigen testing in patients with CAP (table 1).
Microbiology
A bacterial pathogen was identified in 566 (32%) CAP patients and 178 (34%) HCAP patients, most frequently based on sputum culture (n = 368, 50%), urinary antigen testing (n = 224, 30%), blood culture (n = 98, 13%), bronchoalveolar lavage (n = 22, 3%) or serology (n = 13, 2%). The most frequent causative pathogen was S. pneumoniae in both CAP and HCAP patients (14.0% and 11.2%, respectively, table 2). In comparison to CAP, HCAP was less frequently caused by S. pneumoniae and H. influenzae and more frequently caused by S. aureus, P. aeruginosa and E. coli, and multiple pathogens were more frequently identified. Of all the 4,464 bacterial pathogen / antibiotic strategy combinations, 20% (n = 909) were confirmed by susceptibility testing; 65% (n = 2,921) were assumed to be sensitive or resistant, based on intrinsic resistance or national surveillance; and 15% (n = 634) were unknown.
Predictive value for amoxicillin non-susceptibility
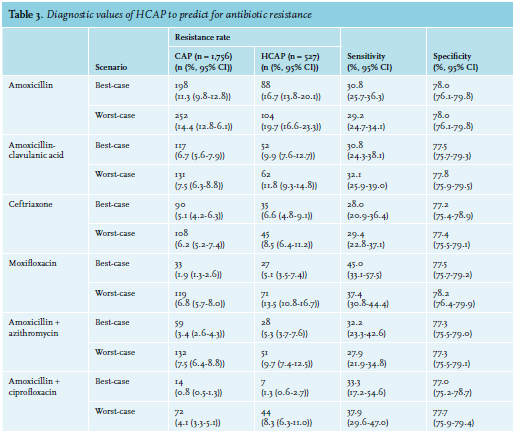
The prevalence of non-susceptibility to amoxicillin for the best-case and worst-case scenarios were 11.3% (95% confidence interval (CI) 9.9%-12.8%) and 14.4% (95% CI 12.9%-16.1%) in CAP patients, respectively, and 16.7% (95% CI 13.6%-20.1%) and 19.7% (95% CI 16.6%-23.3%) in HCAP patients, respectively (figures 1A and B). The corresponding negative predictive values, which are the prevalence of amoxicillin susceptibility in CAP patients without HCAP criteria, were 88.7% (95% CI 87.2%-90.1%) and 85.6% (95% CI 83.9%-87.2%) (1 minus non-susceptibility rate in CAP patients) for the best- and worst-case scenarios respectively, with respective sensitivities of 30.8% (95% CI 25.7%-36.3%) and 29.2% (95% CI 24.7%-34.1%) and specificities of 78.0% (95% CI 76.1%-79.8%) and 78.0% (95% CI 76.1%-79.8%)(table 3).
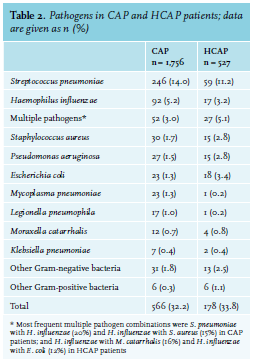
When comparing antibiotic non-susceptibility rates, we used the non-susceptibility rate for amoxicillin as a reference, which was 11.3/14.4% (best-case and worst-case) in CAP patients and 16.7/19.7% (best-case and worst-case) in HCAP patients. In comparison to this reference, other antibiotic combinations reduced the proportion of patients with non-susceptibility by 5-10% (CAP) and 7-16% (HCAP). The largest reduction in non-susceptibility compared to amoxicillin was achieved by adding ciprofloxacin to amoxicillin in both CAP and HCAP patients. In CAP patients, the 11.3/14.4% (best-case and worst-case) non-susceptibility to amoxicillin was reduced by 10% to a non-susceptibility of 0.8% (95% CI 0.4%-1.3%; best-case) and 4.1% (95% CI 3.3%-5.1%; worst-case). In HCAP patients, the 16.7/19.7% (best-case and worst-case) was reduced by 11-16% to a non-susceptibility of 1.3% (95% CI 0.6%-2.7%; best-case) and 8.3% (95% CI 6.3%-11.0%; worst-case) with amoxicillin plus ciprofloxacin (figures 1C and D).
DISCUSSION
Our study focused on patients with a clinical diagnosis of CAP admitted to non-ICU wards. We determined that non-susceptibility of CAP pathogens to amoxicillin was 5-6% higher in patients who met the HCAP criteria compared to patients without HCAP criteria. The most commonly identified pathogens were S. pneumoniae and H. influenzae in CAP patients and S. pneumoniae, multiple pathogens, S. aureus, P. aeruginosa and E. coli in HCAP patients. 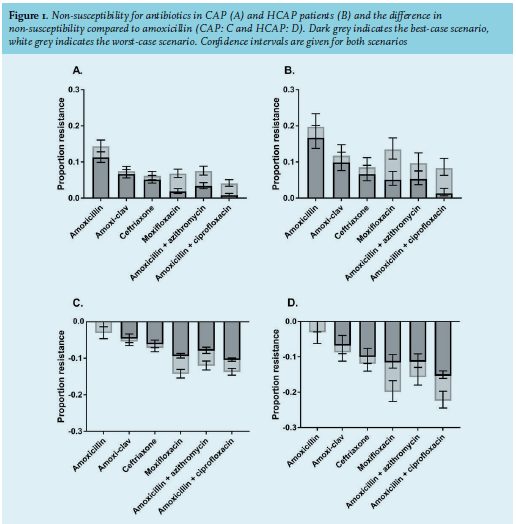
This study has several strengths. We used high quality data from a prospective multicentre trial, including consecutive patients with moderate-severe communityacquired pneumonia, irrespective of whether a bacterial pathogen was isolated. In contrast to including patients with positive cultures only, the predictive values presented here are directly relevant for clinical practice.13,17,20 In addition, we used extensive antibiotic susceptibility data and assessed non-susceptibility over a range of different empirical antibiotic treatment regimens. There were also several limitations. First, patients residing in nursing homes were excluded because their disease was not considered to be CAP. Therefore, one could argue that we did not include the entire spectrum/domain of HCAP and the presented results might not be generalisable to the international HCAP definition. However, these patients would generally be considered as hospital- or nursing-home acquired pneumonia and treated as such. Second, diagnostic testing was performed as part of routine care, which is why blood cultures, sputum cultures and urinary antigen testing were not uniformly performed. Yet, although we cannot exclude the possibility of bias in outcome assessment, there were no major differences between rates of microbiological testing in CAP and HCAP patients. Third, although there were missing susceptibility data for individual antibiotics in certain pathogens, imputed susceptibility data were based on local surveillance data and therefore, generalisable to settings with low antibiotic resistance. In addition, we performed sensitivity analyses on susceptibility patterns that remained unknown with a best-case and worst-case scenario where the unknown susceptibilities were either all susceptible or non-susceptible. These sensitivity analyses yielded only small variations in non-susceptibility for the different antibiotic regimens. Fourth, the probable causative pathogen was, in many cases, based on sputum cultures, which might represent colonisation rather than infection. We therefore only considered plausible pneumonia pathogens in our analyses. Lastly, we assumed cases of pneumonia without a causative pathogen to be susceptible to all antibiotics, which might not be true in case of false-negative culture results for resistant pathogens in a subset of patients. To conclude, HCAP criteria predict for higher non-susceptibility rates to amoxicillin in patients hospitalized with CAP and admitted to non-ICU wards in the Netherlands. However, we consider the absolute risk difference of non-susceptibility to amoxicillin between CAP and HCAP patients as being too low to justify treating all HCAP patients with broad-spectrum antibiotics. Future research should focus on identifying and validating risk factors to predict for narrow-spectrum beta-lactam antibiotic non-susceptibility that are appropriate for settings with low antibiotic resistance. Furthermore, prediction rules need to be evaluated in randomised clinical trials to show benefit on clinical outcome.
ACKNOWLEDGMENT AND DISCLOSURES
The CAP-START trial was supported by a grant (171202002) from the Netherlands Organization for Health Research and Development. Part of these data were presented at the 27th European Congress of Clinical Microbiology and Infectious Disease in Vienna, Austria, from 22-25 April 2017 (#OS0990). MJB: Novartis Europe Advisory Board for Daptomycin; Pfizer Netherlands Advisory Board for vaccines; a grant from Pfizer Netherlands for investigating aetiology of CAP. The other authors declare no conflicts of interests.
REFERENCES