

KEYWORDS
Tyrosine kinase inhibitors, chronic myeloid leukemia, BCR-ABL, coronary artery spasms, off-side inhibitions
CASE SERIES
Patient A
A 54-year-old Caucasian male with CML in chronic phase (CP) was admitted to the ICU of our hospital after a successful resuscitation because of an out-of-hospital cardiac arrest in June 2013. His CML was first diagnosed in January 2005 and treated with imatinib 400 mg o.d. after brief use of hydroxyurea. Due to lack of cytogenetic response, his treatment was switched to dasatinib 50 mg b.d. resulting in a major molecular response (MR3, BCR-ABL ≤ 0.1%). Unfortunately, in July 2010 he developed pleural effusion treated with furosemide, thoracentesis and dose reduction of dasatinib (40 mg b.d.). In January 2013, the pleural effusion relapsed and nilotinib 300 mg b.d. was started in February 2013 while still on a MR 3.
In June 2013, he complained of transient chest pain at the out-patient clinic. A cardiac consultation was planned for the next day and acetylsalicylic acid and metoprolol were directly initiated. However, the following night he collapsed with ventricular fibrillation as first recorded rhythm. After defibrillation, ECGs showed transient signs of anterior wall infarction (figure 1) and normal QTc. A coronary angiography (CAG) was subsequently performed, which showed clear signs of coronary artery spasms (CAS) responsive to intracoronary nitroglycerine and no coronary narrowing related to arteriosclerosis (figure 2). Post-resuscitation cardiac troponin-T showed a typical rise and fall with a maximum of 863 ng/l (normal value: < 14 ng/l). Later that day a new ECG showed ST-elevation corroborative with inferoposterolateral infarction but again normalized after vasodilatory treatment. No other explanation for CAS (hypomagnesemia or known offending agents) was present. So, in a short period of time two different areas of the heart were briefly exposed to severe ischemia in the absence of stenotic coronary artery disease. During the ICU admission nilotinib was temporarily halted. 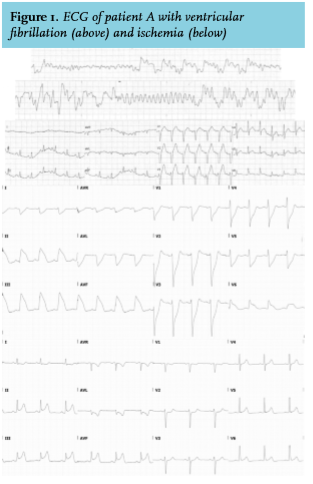
Two years later he experienced a similar episode of acute chest pain, without irregularities on CAG, probably due to CAS this time caused by bosutinib. After optimizing isosorbide mononitrate dosing and bosutinib dose reduction to 300 mg o.d. with persistence of MR4.5 (BCR-ABL ≤ 0.01%) the patient remained free of complaints.
Patient B
Patient B is a 46-year-old Caucasian male, first diagnosed with CML in blast crisis (BC) in January 2013 for which nilotinib 400 mg b.d. was given. In April 2013, he received an allogeneic T-cell depleted stem cell transplantation with a matched unrelated donor after myeloablative conditioning followed by a pre-emptive donor lymphocyte infusion (DLI). In December 2013 he presented with headache, caused by a central nervous system (CNS) CML blast relapse without signs of a systemic relapse. He was treated with liposomal cytarabine intrathecally combined with oral dexamethasone and dasatinib 140 mg o.d. resulting in a complete remission. A second CNS relapse in February 2014 was treated with dasatinib dose increase to 180 mg o.d. and liposomal cytarabine was readministered intrathecally, followed by craniospinal radiation therapy (33 Gy) and therapeutic DLI. After radiation therapy, dasatinib was restarted at a dose of 100 mg o.d. without further evidence of CNS relapse ever since.
Before transplantation ECG analysis indicated signs of a previous inferior wall infarction with pathological Q-waves. Although confirmed by echocardiography, no secondary prophylaxis was started.
In February 2016, he presented with acute chest pain typical for angina pectoris without ECG changes at the cardiac emergency department. Repeatedly, cardiac biomarkers were normal and the patient was discharged for further outpatient analysis. According to guidelines, carbasalate calcium, metoprolol and simvastatin were started. As the patient refused coronary angiogram, conservative therapy was intensified by adding nifedipine. Adenosine stress cardiac MR imaging revealed ischemia in mid-anterolateral and mid-inferior segments without new electrocardiographic changes.
A few weeks later he was again admitted to the coronary care unit with acute chest pain. Now his ECG showed ST-segment depression in V2-V5 with ST-segment resolution after intravenous nitroglycerin (figure 3). His QTc was 431 ms. Cardiac troponin-T showed a typical rise and fall with a maximum of 768 ng/l. It was concluded that the patient suffered a non-STEMI. For risk stratification a CAG was performed. The right coronary artery (RCA) showed an evident ostial lesion, which significantly regressed after intracoronary nitroglycerine. In the left mid-anterior descending artery (LAD) a long trajectory of stenosis (maximum stenosis of 60%) was observed, fractional flow reserve (FFR) assessment of this trajectory showed a significant pressure drop after intravenous adenosine, indicative of ischemia. The circumflex artery was occluded and was considered a chronic occlusion given the rather extensive collateral network from the RCA and LAD. A FFR-guided percutaneous coronary intervention (PCI) of the intermediate lesion mid LAD was performed because of suspected ischemia in the anterolateral wall, also in accordance with the previous stress MR imaging findings. Strikingly, the fractional flow reserve measurement after PCI was actually worse than before, which raised suspicion of a different etiology than a flow-limiting atherosclerotic lesion, i.e. CAS. A switch to conservative medical treatment was decided upon.
Nevertheless, after the PCI the patient experienced recurrent episodes of anginal pain, Canadian Cardiovascular Society class IV, with ST-depression in V2-V5 on ECG. On every occasion he responded quickly to nitroglycerin, without angina pectoris on exertion afterwards. A CAG was repeated showing that the stent in the LAD was patent with normal flow. In the proximal RCA significant spasm occurred again that disappeared after nitroglycerine administration. Despite treatment with verapamil and isosorbide mononitrate his acute chest symptoms kept recurring. It was hypothesized that recurrent vasospasms of the RCA compromised the collateral flow to the circumflex artery causing ischemia. In the absence of hypomagnesemia and satisfactory effects of vasodilatory drugs, an attempt to open the circumflex artery failed. Following the observations in patient A, dasatinib was considered a potential offending agent (CTCAE grade 3).1 In absence of other treatment options of TKI that cross the blood-brain barrier, and in an attempt to reduce symptoms, dasatinib dosage was lowered to 70 mg o.d. With a dasatinib level of 3.38 ug/l (target range 1.4-3.4 ug/L) BCR-ABL1 MR 4.5 persisted.
In September 2016, the patient developed pleural effusion, for which dasatinib was temporarily ceased and prednisolon was started with excellent result. In that period he remained free of anginal complaints, in support of the role of dasatinib with respect to his complaints. Restarting dasatinib immediately induced new episodes of angina pectoris. Anti-vasospasm therapy was intensified by adding nicorandil keeping a MR 4.5 while on dasatinib.
DISCUSSION
In recent years, TKIs have shifted treatment options for patients with CML, with improved prognosis of patients and life expectancy approaching that of the general population.2 However, TKI use causes a range of off-target side effects, some of which are well established, i.e. pleural effusion as encountered in both our patients. These side effects are caused by off-target effects on other tyrosine kinases.3
Cardiac side effects and arterial vascular events including angina pectoris, pericardial effusion, bradycardia, heart failure and QTc prolongation are common due to TKI treatment for CML.4 Nilotinib and especially ponatinib (around 10%) are more frequently associated with cardiac side effects and arterial vascular events than imatinib, dasatinib and bosutinib (2-5%).5-7 The incidence of cardiovascular side effects with TKIs for other indications varies, with higher incidence for VEGF inhibitors, as would be expected, with hypertension in up to 50% of patients and chest pain in 15% of the patients. Other TKIs, like ALK (e.g. crizotinib), JAK2 (e.g. ruxolitinub) and BTK (ibrutinib) inhibitors have low incidence for vascular side effects.8
The pathogenesis of arterial vascular events due to TKI treatment appears to be multifactorial, influenced by known pro-atherogenic risk factors like hyperglycemia and hypercholesterolemia, which are even increased by some TKIs (nilotinib).9,10 Our patients did not develop hyperglycemia or hypercholesterolemia through TKI treatment. TKIs can change proliferation of endothelial cells and angiogenesis by blocking PDGFR and KIT receptor. Discoidin domain receptor 1 (DDR1) is inhibited by TKIs for CML and plays an important role in plaque formation in arteriosclerosis.9,11
Coronary artery spasms have, to our knowledge, never been reported as a side-effect of TKIs in the literature, or to the Dutch medicine evaluation board (Lareb), in contrast to chemotherapeutic drugs such as paclitaxel, capecitabine and gemcitabine.12-14
Coronary artery spasms, also known as vasospastic angina or Prinzmetal angina are caused by focal or diffuse spasm of a coronary artery. This might result in significant temporary obstruction and even myocardial infarction, although short episodes can go unnoticed. The pathogenesis is not entirely understood. Yet, catecholamine activity, chronic inflammation, endothelial dysfunction, availability of nitric oxide, smooth muscle hypercontractibility and activation of the Rho/ROCK pathway seem to play a role.15 Coronary artery spasms can be accompanied by arrhythmias, including ventricular fibrillation, which seldom subsides spontaneously. Known risk factors for spasms are age, smoking (patient B), hypomagnesaemia, alcohol consumption and stress.16 For patient A, none of these risk factors were present.
Sorafenib, a TKI to treat renal cell carcinoma and hepatocellular carcinoma is associated with coronary artery spasms. It exerts its cytotoxic effect by inhibition of Raf, but it also inhibits its downstream effectors MEK and ERK regulating cellular proliferation and survival. Coronary artery spasms induced by sorafenib are possibly due to downstream inhibition of MEK which could cause decreased suppression – and, by consequence, the up-regulation – of the Rho/ROCK pathway.17,18 Activation of the Rho/ROCK pathway is shown to have an important role in the pathogenesis of coronary artery spasms due to augmentation of Ca2+ release.15,17 The Rho/ROCK is also important for cell-cell adhesion. Inhibition of normal c-Abl kinases by STI571 (imatinib mesylate) has been shown to activate the Rho/ROCK pathway and influence cell-cell adhesion.19 Inhibition of normal c-Abl has been suggested as a contributing cause of cardiotoxicity by imatinib.20 Inhibition of BCR-ABL1 and normal Abl-kinases with TKIs could lead to down-regulation of the Raf/MEK/ERK and hence to the same break in suppression leading to up-regulation of the Rho/ROCK pathway (figure 4). There are to our knowledge no reports about CAS and other inhibitors of the Raf/MEK/ERK pathway, like trametinib, vemurafenib and dabrafenib.
First line treatment for coronary artery spasms consists of high dose long-acting calcium channel blockers such as nifedipine 60 mg/day or diltiazem 360 mg/day.21 In severe cases non-dihydropyridine calcium-antagonists can be combined with dihydropyridine calcium-antagonists. Other options include fluvastatin treatment, long acting nitrates or nicorandil.22 The Rho-kinase inhibitor fasudil has also shown to be beneficial in vasospastic angina.23 Other cardio-vascular risk factors for coronary artery spasms, such as smoking, hypomagnesaemia and use of beta blockers should be avoided if possible.16 In cases of ventricular fibrillation an ICD implantation should be considered and ischemic heart disease should be treated properly.24
In our patients, CAS occurred with the use of nilotinib, dasatinib and bosutinib suggesting a class effect of TKIs used for CML. Since the pathogenesis of coronary artery spasms due to TKIs has not been fully elucidated, it is difficult to choose the best TKI when CAS is encountered. Before starting a TKI the prescribing physician should be aware of the possible vascular side effects and the patient’s cardiovascular risk profile. The European CVD risk assessment score has been shown to predict cardiovascular complications in patients during nilotinib treatment and could be used to assess patients at higher risk for vascular complications.25 In patients with higher risks, imatinib in first line or bosutinib for second line should be the TKI of choice, due to low off-target inhibition of other tyrosine kinases and lower rate of vascular side effects. In patients with a higher risk or a cardiovascular history we advise monitoring and treatment of cholesterol, glucose, magnesium and blood pressure on a regular basis, combined with obtaining a thorough medical history focused on cardiovascular complaints. In case of CAS and no suitable alternative for TKI, as in patient B, dose reduction could be considered, since other off-target effects like pleural effusion seem to be dose-dependent.26 Due to the low incidence and unpredictability of CAS, we do not advise prophylactic treatment with calcium channel blockers, even in patients with previous cardiovascular history. Both patients had a moderate risk according to this risk assessment, which in retrospection raises doubts as to whether nilotinib was the best first line treatment in patient A.
CONCLUSION
In conclusion, we describe two CML cases with coronary artery spasms related to nilotinib, dasatinib and bosutinib. When patients on TKI present themselves with chest pain, CAS should be considered. Inhibition of BCR-ABL1 and normal Abl-kinases with TKIs could possibly lead to up-regulation of the Rho/ROCK pathway which could play a causative role. No specific treatment is available and standard treatment of coronary artery spasm, including high dose long-acting calcium channel blockers, should be considered. If clinically possible, a switch of TKI or lowering the dose is advocated (Expert opinion). Imatinib seems to be the TKI of choice since this drug possesses the lowest risk of arterial events, showing similar overall survival and response as other TKI (Grade A evidence).7,27
ACKNOWLEDGEMENTS
We thank Peter Donnelly for critically evaluating our manuscript for English language.
REFERENCES