

KEYWORDS
Bacteraemia, infectious diseases consultation, Staphylococcus aureus
INTRODUCTION
Bacteraemia caused by Staphylococcus aureus is often accompanied by complications and associated with a high mortality rate, even when adequate therapy is given.1-4
The number of cases with Staphylococcus aureus bacteraemia (SAB) has increased over the past 25 years.5,6 Apart from known risk factors such as colonization with S. aureus, (treatment of) malignancy, prior hospitalization within 30 days of onset of illness and surgical wounds or trauma, this may be due to the increasing use of haemodialysis, and (intra-vascular) prosthetic devices.7-11 The frequency of metastatic complications from SAB is high, ranging from 27% to 53%.10,12,13 In community acquired SAB, metastatic complications are more common and tend to be more severe. This is probably due to late presentation, late diagnosis and delayed treatment of the bacteraemia.14,15
Internationally, a number of recommendations have been formulated regarding the management of SAB.16,17 Major priorities are obtaining multiple follow up blood cultures, performing imaging like echocardiography, right choice and sufficient duration of antibiotic treatment, as well as bedside consultation by an infectious diseases specialist of every patient with SAB.18,19 Furthermore, recent developments show that an FDG/PET-CT scan can be helpful to detect metastatic infections of SAB, although this technique has not yet been implemented in international SAB management guidelines.20
In our hospital, the department of medical microbiology recommends to all physicians treating patients with SAB to consult an internist-infectiologist, being the consultant infectious diseases specialist (IDS), in order to improve outcome.
The aim of this study was to compare the management and outcome of SAB between patients with and without bedside IDS consultation and to determine whether the previously noted international guidelines were followed.
MATERIAL AND METHODS
Study design and setting
We conducted a retrospective study in the Maastricht University Medical Centre+ (MUMC+), a tertiary care university hospital in The Netherlands with a capacity of 715 beds and all facilities, including neurosurgery and cardiothoracic surgery.
Patients
All adult patients (18 years or older) with a positive blood culture for S. aureus in the period of 1 January 2010 to 31 December 2013 were included. Exclusion criteria were poly-microbial blood cultures (with the exception of coagulase negative staphylococci (CNS), which were considered to be contamination), lost to follow-up, no antibiotic treatment of the bacteraemia, and no hospital admission.
Data collection
All blood cultures positive for S. aureus were identified with the laboratory registration system Philips Labosys (Philips Medical Systems, Eindhoven, the Netherlands). Standardized case report forms were used to extract the data from the hospital charts. We recorded baseline characteristics, underlying diseases, comorbidities and prosthetic material.
All microbiologic data, including negative blood cultures, were recorded, as was imaging specifically performed for SAB diagnostics. Suspected primary focus of the bacteraemia and metastatic complications (including endocarditis and vertebral osteomyelitis) were based on the conclusion in the patient charts and/or radiological findings. Next to this, choice of antibiotic therapy and duration of treatment were noted, as well as information whether or not an IDS was consulted. Of all patients, a Fowler score was calculated based on clinical variables that describe the probability of complicated SAB, with a maximum of five points. Two points can be given for a positive follow-up blood culture at 48-96 hours, and one point can be given for each of the following: skin examination findings identifying an acute systemic infection, persistent fever at 72 hours and community acquired (CA) SAB.16
Definitions
(CA) SAB was defined as a positive blood culture taken within 48 hours of admission, hospital acquired (HA) SAB as a positive blood culture taken more than 48 hours after admission, and healthcare associated (HCA) SAB as a positive blood culture taken within 48 hours of admission from patients who had been admitted to a hospital or nursing home within the previous three months, patients who were haemodialysis dependent, wore a permanent intravascular catheter, or underwent intermittent chemotherapy.
Uncomplicated SAB was defined as a catheter-related bloodstream infection with negative results of follow-up blood cultures at 48 to 96 hours after starting antibiotic treatment, no persistent fever after 72 hours of therapy, and no signs or symptoms of metastatic infection. Complicated SAB was defined as a bacteraemia with a positive follow-up blood culture with S. aureus at 48 to 96 hours, or persistent fever after 72 hours of therapy, or signs or symptoms of metastatic complications.
Metastatic complications were defined as a positive microbiological culture with S. aureus from a previously sterile site, from abscesses or from removed foreign bodies. In addition, radiographic abnormalities suggesting haematogenous spread of the infection were also classed as metastatic complications.
Consultation by an infectious disease specialist was defined as consultation for the purpose of management of SAB during the initial hospitalization. If SAB developed while infectious diseases consultation was already in place for another reason, the date of first positive blood culture was taken as the consultation initiation date.21 The choice of antibiotic treatment was defined as adequate when the cultured S. aureus isolate was susceptible to the chosen antibiotic therapy.
Our local Dutch guidelines are similar to the international guidelines. Both the Infectious Disease Society of America (IDSA) and the Dutch Working Party on Antibiotic Policy (Dutch acronym: SWAB) recommend a four to six week treatment for complicated SAB, depending on the kind of (metastatic) complication. Uncomplicated SAB can be treated by 14 days of adequate antibiotic therapy, at least seven days intravenously. Furthermore, major priorities are obtaining multiple follow up blood cultures, performing imaging such as echocardiography, and the appropriate choice and sufficient duration of antibiotic treatment.22,23
Therapy failure was defined as a positive blood culture with S. aureus for 10 or more days after the start of adequate antibiotic therapy, or relapse of infection, which was defined as a positive blood culture with S. aureus within 12 weeks after the completion of antibiotic therapy.
Statistical analysis
Statistical analysis was performed with IBM SPSS version 21 (SPSS Inc., Chicago, IL, USA) software. Continuous variables were reported as median with interquartile range (IQR), and categorical variables as proportions. Comparisons between two groups were done using a Mann-Whitney test for continuous data and the Fisher exact test for categorical data. The log-rank test was used to test for differences in survival. Multiple regression analysis for mortality was performed with variables of clinical importance for mortality, such as risk factors for complicated SAB, imaging (TEE and FDG-PET/CT) and IDS consultation. P-values < 0.05 were considered statistically significant.
Ethical approval
This study was reviewed and approved by the Medical Ethical Committee of the Maastricht University Medical Centre+.
RESULTS
During the four year study period, a total of 826 positive blood cultures with S. aureus were identified with the laboratory registration system, representing 333 SAB episodes. Of these 333 SAB episodes, 99 episodes were excluded, due to an age < 18 years (n = 43), poly-microbial blood culture (n = 31), no admission (n = 3), no treatment (n = 8), death before blood cultures became positive (n = 11) and lost to follow-up (n = 3). After exclusion, 234 episodes of SAB of 234 patients remained. Of these 234 episodes, 59 (25.2%) were community acquired, 101 (43.2%) hospital acquired, and 74 (31.6%) healthcare associated (figure 1).
Table 1 shows the baseline characteristics of the 234 patients included. In total, more males (65%) than females were diagnosed with SAB (152 vs. 82). Consultation by an IDS during the bacteraemia took place in 182 (77.8%) patients. Median time to consultation after drawing blood cultures that first yielded S. aureus was three days with an interquartile range of two to five days (table 2). Patients with a community acquired SAB were consulted by an IDS more frequently.
Management The number of IDS consultations increased in the period from 2010 to 2013; from 58.8% in 2010 to 95.2% in 2013 (figure 2). Management of patients with IDS consultation was more often in accordance with guidelines, compared to patients without consultation. Follow-up blood cultures were obtained more frequently from consulted patients, and they more often underwent diagnostic procedures such as TTE (transthoracic echocardiography), FDG/PET-CT scans, and/or MRI scans. Furthermore, the consulted group was more often treated adequately with respect to both duration and choice of antibiotic treatment (e.g. no addition of gentamicin in prosthetic valve endocarditis was defined as inadequate), compared to those without consultation (156/176 (88.6%) vs. 29/49 (59.2%), p = < 0.001) (table 2).
Management
The number of IDS consultations increased in the period from 2010 to 2013; from 58.8% in 2010 to 95.2% in 2013 (figure 2). Management of patients with IDS consultation was more often in accordance with guidelines, compared to patients without consultation. Follow-up blood cultures were obtained more frequently from consulted patients, and they more often underwent diagnostic procedures such as TTE (transthoracic echocardiography), FDG/PET-CT scans, and/or MRI scans. Furthermore, the consulted group was more often treated adequately with respect to both duration and choice of antibiotic treatment (e.g. no addition of gentamicin in prosthetic valve endocarditis was defined as inadequate), compared to those without consultation (156/176 (88.6%) vs. 29/49 (59.2%), p = < 0.001) (table 2).
Complications and relapse
The consulted group was more often diagnosed with complicated SAB, (108/182 (59.3%) vs. 17/52 (32.7%), p = 0.001). Likewise, the Fowler score was higher (median 1 day 0-2 vs. 0 days 0-1, p = 0.002), and metastatic complications were more frequently detected (82/182 (45.1%) vs. 10/52 (19.2%), p = 0.001). There was no difference in therapy failure or relapse of infection, although there were only few relapses (table 2).
Seven patients had a relapse of SAB. Table 3 shows the characteristics of these seven patients. During the initial SAB, five of these patients were consulted by an IDS (5/182; 2.7%), all of whom were treated adequately, and echocardiography was performed in all (1/5 TEE; 4/5 TEE).
Mortality
Despite the higher number of detected metastatic complications, the 30-day mortality was lower in the consulted group, compared to the group without consultation (22/182 (12.1%) vs. 12/52 (23.1%), p = 0.04). This was confirmed in the multivariate regression analysis (table 4). In addition, performing an FDG/PET-CT scan was also associated with lower mortality. As expected, higher age and ICU admittance were associated with higher mortality.
DISCUSSION
In this evaluation of the management of SAB, we have shown that routine bedside IDS consultation in patients with SAB is associated with better adherence to treatment guidelines and a lower overall mortality rate. Some key diagnostic priorities in the management of SAB, such as follow up blood cultures and echocardiography in every patient with SAB, were more frequently performed where an IDS was consulted. This resulted in a higher detection rate of disseminated disease, but not in an increased mortality rate. This is possibly due to more adequate management and treatment of patients with IDS consultation. Subsequently, as patients without IDS consultation were more often treated inadequately, one would also expect more relapses in this group. However, only a low number of relapses (7/234, 3.0%) was detected in this study. Echocardiography is routine diagnostic care in patients with complicated SAB. FDG/PET-CT scans, however, are not (yet) a standard recommendation in the management of patients with complicated SAB.17 Nevertheless, the implementation of an FDG/PET-CT scan as a standard procedure for all patients with complicated SAB and risk factors for dissemination is advocated, as FDG/PET-CT scanning detects more disseminated disease.13,20 Also, performing an FDG/PET-CT scan was associated with lower mortality in our study. In patients consulted by IDS a higher number of metastatic complications was detected. This is most probably due to the better adherence to guidelines by the consulted IDS. Since follow up blood cultures were obtained more often and more diagnostic procedures (echocardiography, FDG/ PET-CT scans, and MRI scans) were performed, more metastatic complications actually came to light. Another reason could be that patients who were more severely ill were consulted by an IDS more frequently. This bias could not completely be evaded, bedside IDS consultation has been implemented routinely in all patients with SAB in our hospital, particularly in recent years (95.2% of patients with SAB in 2013).
The number of patients who were consulted by an IDS rose during the years studied, from 58.8% in 2009 to 95.2% in 2013. This is probably due to the expansion of the infectious disease specialist team at the end of 2011. Furthermore, stricter rules were agreed upon between the internist-infectiologists (IDS) and the medical microbiologist concerning infectious diseases consultations. These included making the internistinfectiologist aware of all blood cultures positive for S. aureus, so these patients received IDS consultation whether the treating physician asked for it or not. Also, ICU patients and patients in the hematologic ward, who were previously consulted by the medical microbiologist by telephone, from then on received a bedside consultation by the IDS.
Median time to bedside consultation, after the blood cultures which were to yield S. aureus were drawn, was three days. This delay was caused by the fact that the bacteria in the culture need to grow to be noticed, which is often after one to two days. However, a contributing factor to an even longer delay in some patients could be that no bedside consultations were done in the weekends. In the last years of the study, with expansion of the IDS team and strict bedside consultations in almost all patients, time to consultation decreased compared to the early years of the study (2010-2011: median 4 days, mean 5 days, 2012-2013: median 3 days, mean 3 days). 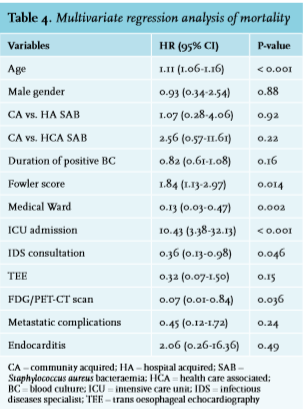
Despite the higher number of metastatic complications in the group with IDS consultation, the 30-day mortality was lower (12.1% vs. 23.1%, p = 0.04), as was confirmed in our multivariate analysis and previously has been shown in other studies.11,14,24-26
There are some limitations to our study. First, this being a single centre retrospective study, we nonetheless included almost 250 patients in the four year study period. Furthermore, no distinction was made between the separate components of the IDS consult such as recognition of endocarditis stigmata, correctly obtaining follow-up blood cultures and diagnostics, and appropriate and adequate administration of antibiotics. Next to this, we had to rely on the completeness of the charts. During the last three years of the study period, the hospital used electronic patient files, which improved the availability of data. In addition, the investigators were not blinded to IDS consultation. Therefore, we used strict definitions to minimize any potential bias. Another limitation might be that patients who were discharged in a clinically good condition, may have developed metastatic complications or a relapse at home without referral to our clinic. Since our hospital serves both as a secondary and tertiary referral centre, follow-up after discharge will most likely be done in our hospital, but we can’t rule out that a SAB complication was treated in another hospital and was therefore missed in our analysis.
In conclusion, our study shows the need for bedside IDS consultation in every patient with SAB. Consultation by an IDS results in better adherence to management guidelines and detection of more metastatic complications with better overall survival. Therefore, in patients with SAB, bedside consultation by an IDS should be obligatory.
ACKNOWLEDGMENTS
We want to thank the ICU and the Medical Archives for their contributions to the data collection, and John Penders of the department of Medical Microbiology for his help with the statistical analysis.
DISCLOSURES
All authors declare no conflict of interest. No funding or financial support was received.
REFERENCES