

KEYWORDS
Dementia, ICD codes, hospital register, prognosis, validity
INTRODUCTION
Dementia is one of the major causes of morbidity and mortality among older people. There is a great need for reliable methods to elucidate mechanisms and risk factors underlying the poor prognosis of dementia and to give insights into morbidity and mortality risks to reduce this burden. As a national dementia registry is not available in most countries, evidence on morbidity and mortality risks is only available from small specific population studies.1,2 These studies have limited precision, though, and generalisability may be questioned. Therefore, confirmation from large long-term population-based studies is needed. However, those studies are complex, expensive and time consuming, especially in this population where loss to follow-up is common as a result from accelerated cognitive decline and mortality.3,4 The potential of using linkage methods to create large disease-specific cohort studies is increasingly being recognised. Existing nationwide administrative registries and databases are linked which enables estimation of, for example, age-sex specific mortality rates in an efficient and relatively inexpensive way. The validity of the outcomes from studies using these data, however, depends on the completeness and accuracy of the data (both disease status as well as disease outcome event (cause of hospitalisation and cause of death)) in the national registers and the accuracy of the linkages. The validity of the diagnosis of dementia in national registries is largely unknown. Therefore, we aimed to assess the validity of dementia diagnoses in the Dutch nationwide Hospital Discharge Register (HDR). In the HDR, medical and administrative data of inpatients and day clinic patients visiting Dutch hospitals are routinely recorded.5 The results from this study will provide information about the usefulness of the HDR in future nationwide research on the prognosis of dementia.
METHODS
Dutch Hospital Discharge Register
Since the 1960s, medical and administrative data of admitted and day clinic patients visiting a Dutch hospital are recorded in the HDR; no information on outpatient visits is available. Circa 100 hospitals participate in the register. The HDR contains information on patient demographics, principle and secondary diagnoses, and other admitting and discharge data. At the medical administration department of each participating hospital, a new record is created after each new hospital admission or day clinic visit by a professional clinical coder based on admission data and the discharge letter. The principle and secondary diagnoses are determined and coded using the ninth revision of the International Classification of Diseases – Clinical Modification (ICD-9-CM).6 Although investigators might consider ICD coding of causes for hospitalisation as inferior, there is sufficient evidence to suggest that the coding in the Netherlands is of a high level. It has been shown that 99% of the personal, admission and discharge data, and 84% of the principal discharge diagnoses (validated through medical record review by medical specialists) were correctly registered in a random sample of all hospital admissions registered in the HDR.7 Positive predictive values (PPVs) of registration in the HDR have shown to be 97% for acute myocardial infarction, 95% for subarachnoid haemorrhage, 91% for intracerebral haemorrhage, 98% for non-ruptured abdominal aortic aneurysm (AAA), 97% for ruptured AAA and 80% for congestive heart failure.8-10
Cohort enrolment
We used a random sample of 340 patients aged 40 years and older registered with a principal or secondary diagnosis of dementia (ICD-9 code 290.0; 290.1; 290.3; 290.4; 294.1; 331.0; 331.1; 331.82) in the HDR of the University Medical Center Utrecht between
1 January 2006 and 31 December 2010. The University Medical Center Utrecht is one of the largest healthcare facilities in the Netherlands. The day/memory clinic serves as a secondary and tertiary care referral centre. Patients are referred to the day/memory clinic either in case of memory-related disorders (memory clinic) or with multi-morbidity which might also include memory-related disorders (day clinic). The study was conducted in accordance with privacy legislation in the Netherlands.
Data collection
Information on each patient acquired from the HDR included: patient hospital number, age, gender, admission date, type of hospital contact (admission vs. day clinic) and the principal and secondary diagnoses according to the ICD-9 code. Medical records of the hospital wards (admissions) and memory/day clinic (day clinic visits) that belong to the selected patients were reviewed. Information on each patient acquired from these medical records included: diagnosis made by the physician, medical history/comorbidity and medication use/polypharmacy. To obtain an overview of the medical history of all patients, presence of comorbidity according to the Charlson Comorbidity Index (CCI) was collected from the medical records using Deyo’s coding algorithm.11 This index does not completely cover comorbidity, but contains 17 major comorbidities. All comorbidities were defined dichotomously (yes or no). Use of medication was evaluated to assess the presence of polypharmacy. Polypharmacy was defined as the use of five or more regular drugs, excluding temporary drugs (e.g. antibiotics).12
Validation
Diagnosis of dementia reported in the medical records by the treating physician was considered the reference diagnosis. Routine clinical care in all patients who visited the day clinic comprised a standardised diagnostic work-up including a comprehensive geriatric assessment, neurological and physical examination, blood tests and on indication neuropsychological testing. Patients who visited the memory clinic also underwent a standardised extensive neuropsychological assessment. If there was an indication for neuroimaging, patients underwent a computed tomography or magnetic resonance imaging scan. Diagnosis of dementia, and its subtypes, was made at a multidisciplinary consensus meeting based on internationally accepted criteria.13-17 All patients admitted to the geriatric ward received a similar comprehensive geriatric assessment. Patients underwent neuroimaging and a standardised extensive neuropsychological assessment on indication. Patients admitted to other departments where formal cognitive testing was not a routine did not receive a comprehensive geriatric assessment. The majority of these patients had a history of dementia and were consequently coded with a secondary diagnosis of dementia. It was not possible to determine whether these patients had previously undergone formal cognitive testing.
Statistical analysis
Continuous data were summarised as mean and standard deviation or as median and interquartile range where appropriate. Categorical data were summarised as proportions. First, we determined whether patients were correctly classified in the HDR as having dementia (overall dementia; any dementia disorder). Positive predictive values (PPVs) were calculated with 95% confidence intervals (CIs) and defined as the number of patients diagnosed with dementia based on the medical records, divided by the total number of patients coded with dementia in the HDR. Differences between PPVs were analysed by the chi-square test or Fisher’s exact test where appropriate. Multivariate logistic regression models were used to evaluate determinants of inaccuracy in diagnoses of dementia in the HDR. The determinants included in the multivariate models were age, gender, and comorbidity (CCI). In a second multivariate model, comorbidity was replaced by polypharmacy (comorbidity and polypharmacy were not included simultaneously, because these variables were highly correlated). Secondly, we evaluated the accuracy of the two most common dementia subtypes, Alzheimer’s disease (AD) and vascular dementia (VaD), in the HDR. An ICD-9 code for mixed-type dementia (most common is a combination of AD and VaD) does not exist; therefore, patients diagnosed with mixed-type dementia at the hospital ward or memory/day clinic were considered correctly classified in the HDR if they received the following codes: 331.0 (AD), 290.40 (VaD) and 290.0 (senile dementia).
During the validation procedure, we noticed that a large number of the patients registered with ICD-9 code 290.0 (senile dementia) in the HDR were diagnosed with AD according to the treating physician. Therefore, we additionally studied whether patients registered with ICD-9 code 290.0 in the HDR were representative for patients with AD. We calculated the PPV of ICD-9 code 290.0 for the diagnosis of AD. As in future the ICD-9 code 290.0 in the HDR might be used (in addition to ICD-9 code 331.0) to answer prognostic research questions concerning AD, we performed multivariate logistic regression analysis to assess whether there were differences with regard to prognostic determinants between patients registered with ICD-9 code 290.0 in the HDR with and without the reference diagnosis of AD (or mixed-type dementia) according to the treating physician, in a similar approach as described before. Since AD and VaD are the most common subtypes of dementia and since numbers of other dementia subtypes were rather low we only evaluated the validity of AD and VaD. Data were analysed using the SPSS 20.0 statistical package (SPSS Inc, Chicago, Illinois, USA). A two-sided p value < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
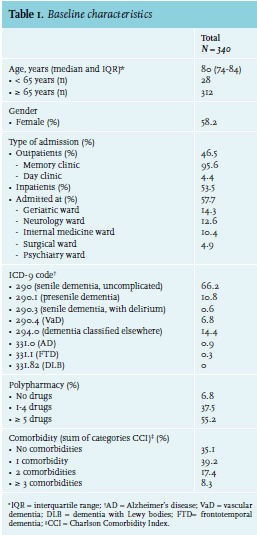
In total 340 medical records of patients admitted between 2006 and 2010 were used in this study. Patient characteristics are shown in table 1.
Validation procedure
Overall dementia
Overall dementia was present in 317 patients (PPV 93.2%; 95% CI 90.0-95.5) based on the reference diagnoses of the treating physicians (table 2). There was no significant difference in PPV for men vs. women: 91.6% (95% CI 85.7-95.2) and 94.4% (95% CI 90.2-97.0) respectively (p = 0.29). The PPV significantly increased with age: 67.9% (95% CI 49.2-82.2) for patients < 65 years (n = 28) vs. 95.5% (95% CI 92.6-97.4) for patients ≥ 65 years (n = 312) (p < 0.01). Furthermore, analyses showed no difference in PPV regarding the setting of diagnosis (admission versus memory/day clinic visit), number of comorbidities and presence of polypharmacy. Multivariate analysis showed similar results with a significantly higher probability of an accurate diagnosis for patients ≥ 65 years compared with patients < 65 years (adjusted odds ratio (OR) 10.5; 95% CI 3.7-30.3).
Alzheimer’s disease
In total 228 patients were registered with either ICD-9 code 290.0 (senile dementia) or 331.0 (AD) in the HDR. Three patients were diagnosed with ICD-9 code 331.0, all correctly classified as AD according to the reference diagnosis reported by the treating physician (PPV 100%). Of the 225 patients registered with ICD-9 code 290.0, 141 patients were diagnosed with AD according to the reference diagnosis reported by the treating physician (PPV 62.7%; 95% CI 56.2-68.7). In total 144 of the 228 patients with either ICD-9 code 290.0 or 331.0 were correctly classified as AD, which resulted in a PPV of 63.2% (95% CI 56.7-69.2%) (table 3). Diagnoses in the HDR from memory/day clinic patients were more accurate compared with diagnoses in the HDR from admitted patients (PPV 78.2%; 95% CI 69.8-84.7% vs. 46.8%; 95% CI 37.7-56.1% (p < 0.01)). There were no significant differences in accuracy between the wards (geriatric versus other wards). Other variables did not significantly affect the accuracy.
In total 84 of the 225 patients (37.3%) registered in the HDR with ICD-9 code 290.0 did not have AD according to the reference diagnoses reported by the treating physicians. Of these 84 patients, 36 (42.9%) were diagnosed with dementia not otherwise specified according to the treating physicians, 22 patients (26.2%) with VaD, ten patients (11.9%) with dementia with Lewy bodies, five patients (6.0%) with frontotemporal dementia, five patients (6.0%) with Parkinson’s dementia, one patient (1.2%) with mild cognitive impairment and five patients (6.0%) were not demented.
As in future research the ICD-9 code 290.0 in the HDR might be used (in addition to ICD-9 code 331.0) to answer questions on prognosis concerning AD, prognostic determinants (age, gender, comorbidity) were assessed to see whether there were differences between patients registered in the HDR with ICD-9 code 290.0 with versus without the reference diagnosis of AD (or mixed-type dementia), according to the treating physician. This multivariate analysis showed no statistically significant differences in age, sex and comorbidity between patients with the reference diagnosis of AD (or mixed-type dementia) and patients without this reference diagnosis. Similarly, a multivariate analysis was performed using polypharmacy as a covariate instead of number of comorbidities. This analysis showed that patients with the reference diagnosis of AD (or mixed-type dementia) were less likely to have polypharmacy compared with patients without this reference diagnosis (adjusted OR 0.5; 95% CI 0.3-0.9). Polypharmacy was present in 47.5% of the patients with the reference diagnosis of AD compared with 61.9% of the patients without the reference diagnosis of AD.
Vascular dementia
In total 23 patients were registered with VaD in the HDR (ICD-9 code 290.40). According to the reference diagnoses reported by the treating physicians, two patients were improperly classified as VaD patients, resulting in a PPV of 91.3% (95% CI 72.0-98.8%)
(table 3). There were no significant differences in PPV according to age, gender, setting of diagnosis, and comorbidity. All patients had polypharmacy.
DISCUSSION
The results in this study indicate that the validity of using HDR codes to identify patients with dementia is high. Overall PPV was 93.2%. The accuracy was neither influenced by gender and setting of diagnosis (admission or day/memory clinic) nor by number of comorbidities and polypharmacy. Multivariate analysis showed a significantly lower validity in patients younger than 65 years versus those older than 65 years, which is in line with a previously performed study reporting on over-registration of dementia in relatively young patients.18 Overestimation might result from a broader differential diagnosis of dementia in younger patients. Often, extensive diagnostic strategies and much longer time are needed before definite confirmation of diagnosis of dementia is possible. Consequently, in younger patients who are registered with dementia in the HDR, the medical files from the doctor more often reveal conversion of dementia diagnosis to another diagnosis. Our results are consistent with two previously performed studies in Northern Europe, showing PPVs of dementia discharge diagnosis close to and more than 90%.19,20
Accuracy regarding registered dementia subtype diagnoses was also high, but less reliable. Although PPV for code 290.4 (VaD) and code 331.0 (AD) was 91.3% and 100% respectively, the numbers of patients within these groups were unexpectedly low (23 and 3 respectively), while AD and VaD contribute to the two most common causes of dementia worldwide.21 During the validation procedure, we noticed that a large number of the patients diagnosed with AD according to the treating physician were registered as senile dementia (ICD-9 code 290.0). Similar findings were reported by Phung et al.19 This could be explained by the fact that the specific subtype of dementia is often diagnosed in a two-step procedure:22 1) identification of dementia (syndrome) during the first visit and
2) identification of the underlying disease (i.e. subtype of dementia) during follow-up visits, usually at the outpatient clinic after additional investigations, such as neuropsychological testing and imaging. In many cases the final diagnosis, including an underlying disease, has therefore not been made at the first visit/discharge. As a consequence, the discharge diagnosis is coded as senile dementia (ICD 290.0). Since follow-up data of outpatient visits are not available in the HDR, the ICD-9 code will not be adjusted after the conclusive diagnosis is reached. Furthermore, in traditional literature senile dementia is often used when referring to AD.23 For this reason, clinical coders might choose to register AD diagnoses with ICD-code 290.0. Both explanations might contribute to the high number of diagnoses registered with ICD-9 code 290.0.
We additionally studied whether patients registered with ICD-9 code 290.0 in the HDR were representative for patients with AD. PPV was modest at 63.2% but overall comparability with respect to prognostic determinants between patients registered in the HDR with ICD-9 code 290.0 with versus without the reference diagnosis of AD (or mixed-type dementia) was high. This implies that the ICD-9 code 290.0 (in addition to ICD-9 code 331.0) can be used to select a representative group of patients in further research with the focus on the prognosis of AD. The validity of diagnosis of AD in the HDR (codes 290.0 and 331.0) from patients of the day/memory clinic was superior to the validity of diagnosis of AD in the HDR from admitted patients (p=0.0001). Admitted patients tend to have higher incidences of delirium and associated symptoms which could be (incorrectly) registered by clinical coders as ICD-9 code 290.0. Secondly, since hospital admissions are more often associated with multiple diagnoses and procedures, the primary and secondary diagnosis might be a matter of opinion of the clinical coder, creating the potential for inaccuracy.24
Strengths
This is one of the few validation studies about dementia registration in the HDR and is therefore of great value for further research on the prognosis of dementia. We had access to all medical journals of the included registered patients with dementia between 2006 and 2010 to validate the HDR. Furthermore, the distribution of dementia diagnoses in this study reflects the general distribution of dementia worldwide, which makes it a representative sample for further research.21
Limitations
The present study showed data from one university hospital in the Netherlands, which may impede nationwide generalisability. However, diagnoses are routinely registered by clinical coders at the medical administer department of a hospital (either academic or not academic) in accordance to a structured procedure using the predefined ICD-9 codes. A previous study that studied the general validity of the HDR using data from 55 hospitals (and coders) showed that 84% of registered diagnoses were correct.7 Thus, the potential of problems with generalisability is less likely. Furthermore, even in small hospitals without special geriatric or elderly care, generalisability is not jeopardised since we found no significant differences in validity between the different wards (geriatric, neurology, internal medicine, surgical, or psychiatric). Diagnosis of dementia in a small hospital will then also be made by other doctors such as neurologists and psychiatrists which will not influence the accuracy.
Clinical implications
This study underlines the potential use of HDR data in future research. Although the HDR does not contain data on outpatients, it is a valid and useful registry of inpatients and day clinic patients. ICD codes 290.0; 290.1; 290.3; 290.4; 294.1; 331.0; 331.1; 331.82 can be used for initial case finding to construct a nationwide cohort of dementia patients, hospitalised and/or memory day clinic domain. Several important questions concerning the prognosis can be answered, since there is a great need to elucidate differences in prognosis of patients with dementia. With the use of a nationwide cohort of dementia patients, short- and long-term morbidity and mortality risks can be assessed and changes over time can be explored. We showed the potential to use the ICD code 290.0 in combination with ICD code 331.0 to identify AD patients although PPV was lower. Furthermore, PPV for VaD diagnosis was shown to be high.
CONCLUSION
In conclusion, we found that the validity of using HDR codes to identify patients with dementia is high. Furthermore, we showed the potential of using the ICD-9 code 290.0 (senile dementia) to select a representative group for AD patients although PPV was lower. Overall, the HDR constitutes a very useful starting point for nationwide research on the prognosis of dementia.
ACKNOWLEDGMENTS
This study was supported by Alzheimer Nederland (project no WE.03-2012-38).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES